On-farm culture (OFC) is based on the theory that clinical mastitis caused by Gram-negative pathogens does not require antimicrobial treatment. In OFC protocols, cows with mastitis have an aseptic milk sample taken, which is incubated on agar or in broth. Antimicrobial treatment is withheld in samples with pure growth of Gramnegative species, or no significant growth. In samples with Grampositive species, mixed or contaminated growth, intramammary antimicrobial treatment is given on label.
Several OFC studies have shown that withholding treatment in cases of mastitis caused by Escherichia coli has no detectable impact on treatment outcome (Lago et al, 2011; Vasquez et al, 2017; Fuenzalida and Ruegg, 2019; Schmenger et al, 2020). One criticism of these studies is that they lack statistical power to detect a meaningful clinical difference in cure rate, i.e. the sample size is too small. Two independent studies in the USA have shown a 10–11% numerical reduction in bacteriological cure risk, which was not ‘statistically significant’ (Lago et al, 2011; Fuenzalida and Ruegg, 2019). A recent study in Germany showed more convincing evidence that bacteriological cure rate is unaffected by OFC: 75.3% with conventional therapy compared with 78.4% in the OFC group (Schmenger et al, 2020). Another USA study found similar days to clinical cure, although bacteriological cure was not assessed (Vasquez et al, 2017).
All trials referenced have shown a significant reduction in antimicrobial use, ranging from 56–73%. However, from a financial perspective, OFC may only be cost-effective on farms with a high prevalence of Gram-negative species. One study estimated that Gram-positive species would have to account for <20% of mastitis cases for most farms to break even (Down et al, 2017). The last large-scale study in the UK estimated that 35% of clinical mastitis was caused by Gram-positive species, 21% by Gram-negative species, with mixed growth in 4.2% and no bacterial growth in 26% of cases (Bradley et al, 2007).
Accuracy of on-farm culture
When using OFC it is important that mastitis caused by Gram-positive pathogens is not missed, as these infections are likely to benefit from treatment. Table 1 shows reported sensitivity, specificity and predictive values of the most commonly available kits on the market. Depending on the kit used, and the time to interpretation, between 15–40% of Gram-positive species could be misidentified.
Table 1. Test performance in identifying Gram-positive pathogens
| Product | Time to reading (hours) | Sensitivity | Specificity | PPV | NPV | Reference |
|---|---|---|---|---|---|---|
| VétoSlide | 24-48 | 80.7% | - | 80% | 82% | Malcata et al, 2019 |
| VétoRapid triplate | 24-48 | 74.3% | - | 70% | 82% | Malcata et al, 2019 |
| Minnesota Easy Culture biplate | 18-24 | 80-85% | 79-87% | 92-85% | 58-64% | Royster et al, 2014 |
| Minnesota Easy Culture triplate | 18-24 | 80-86% | 76-93% | 91-97% | 62-66% | Royster et al, 2014 |
| Petrifilm | 24 | 85.2% | 75.4% | 65.1% | 90.4% | Mansion-de Vries et al, 2014 |
| MastDecide | 12 | 58.6% | 97.0% | 94.4% | 73.2% | Leimbach and Krömker, 2018 |
| 16 | 83.6% | 94.1% | 92.4% | 87.0% | Leimbach and Krömker, 2018 |
The majority of kits are designed to be read at 24 hours (compared with 48 hours recommended by the National Mastitis Council, 2017, or 72 hours reported by some laboratories). The MastDecide test kit is designed to be read between 12–16 hours: although at 12 hours the kit is less accurate at diagnosing Gram-positive infections. Most farms do not have a window of 16 hours between the end of one milking and the start of the next (including time to run the test), so it is impractical to use this kit for making a diagnosis before the next milking.
Some kits are marketed to differentiate specific mastitis pathogens, for example Streptococcus spp. or Staphylococcus aureus. However, few studies have evaluated the accuracy of test kits beyond classification of Gram-positive and negative species. Test performance of the Minnesota Easy Culture triplate in identifying some key pathogens is given in Table 2 (Royster et al, 2014). Despite Streptococcus uberis accounting for 18% of mastitis cases in this study, it was diagnosed in fewer than 1% of observations. Given these results, it is not currently recommended to use OFC kits for making a diagnosis beyond Gram-positive versus negative bacteria.
Table 2. Test performance in identifying clinically important pathogens
| Pathogen | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Escherichia coli | 60% | 95% | 54% | 97% |
| Klebsiella spp. | 33% | 95% | 32% | 97% |
| Staphylococcus aureus | 70% | 94% | 61% | 97% |
| Streptococcus uberis* | 7% | 99% | 50% | 83% |
Figures based on the average of two observers in the study by Royster et al, 2014
*In this study only one observer identified any S. uberis, and so figures have been given for ‘Reader 2’
What about Gram-positive bacteria that do not need treating?
Non-aureus Staphylococci and Corynebacterium spp. are commonly identified in milk samples from cows with mastitis, although they are typically classified as minor udder pathogens (Watts, 1988). Where present, they are associated with a mild increase in cell count, without causing clinical mastitis and with no effect on milk yield (Schukken et al, 2009). For these reasons, cows infected with minor pathogens are unlikely to benefit from treatment. If OFC is only used in clinical cases this will result in no more antimicrobial use than conventional approaches, however if farmers are using OFC instead of somatic cell count to make decisions at drying off, the presence of a Gram-positive bacteria may lead to unnecessary use of antimicrobials.
What about Gram-negative bacteria that might need treating?
The evidence that Gram-negative bacteria do not benefit from treatment is far from conclusive. Historic studies have largely evaluated the effect of parenteral, rather than intramammary treatment (Pyörälä et al, 1994; Erskine et al, 2002; Suojala et al, 2010). The resolution of experimentally induced E. coli mastitis has been shown to be high without treatment (Leininger et al, 2003); however, several studies have diagnosed persistent mastitis caused by Gram-negative bacteria (Döpfer et al, 1999; Bradley and Green, 2000; Fuenzalida and Ruegg, 2020). Though not licensed in the UK, broad spectrum treatment with intramammary ceftiofur has been associated with an increased bacteriological cure of Klebsiella spp. (Schukken et al, 2011; Fuenzalida and Ruegg, 2019). The same studies showed conflicting results for mastitis caused by E. coli: improved cure (Schukken et al, 2011), and no difference (Fuenzalida and Ruegg, 2019).
Commercial test kits lack sensitivity to differentiate E. coli and Klebsiella spp., and so caution should be taken on farms with a higher prevalence of Klebsiella spp. — this should include farms bedding on recycled manure solids (Leach et al, 2015). More research is needed into the risks or benefits of withholding treatment in cases of mastitis caused by other Gram-negative species including Serratia and Pseudomonas spp.
Compliance
In the UK, the majority of mastitis cases, in the author's experience, are diagnosed and treated by farmers, based on protocols defined in a herd health plan. OFC adds complexity to mastitis treatment protocols, and so it is important to monitor farmer compliance. Most studies have been undertaken in a research setting, however in a recent study by Schmenger et al (2020), farmers complied with the treatment protocol in only 44% of cases.
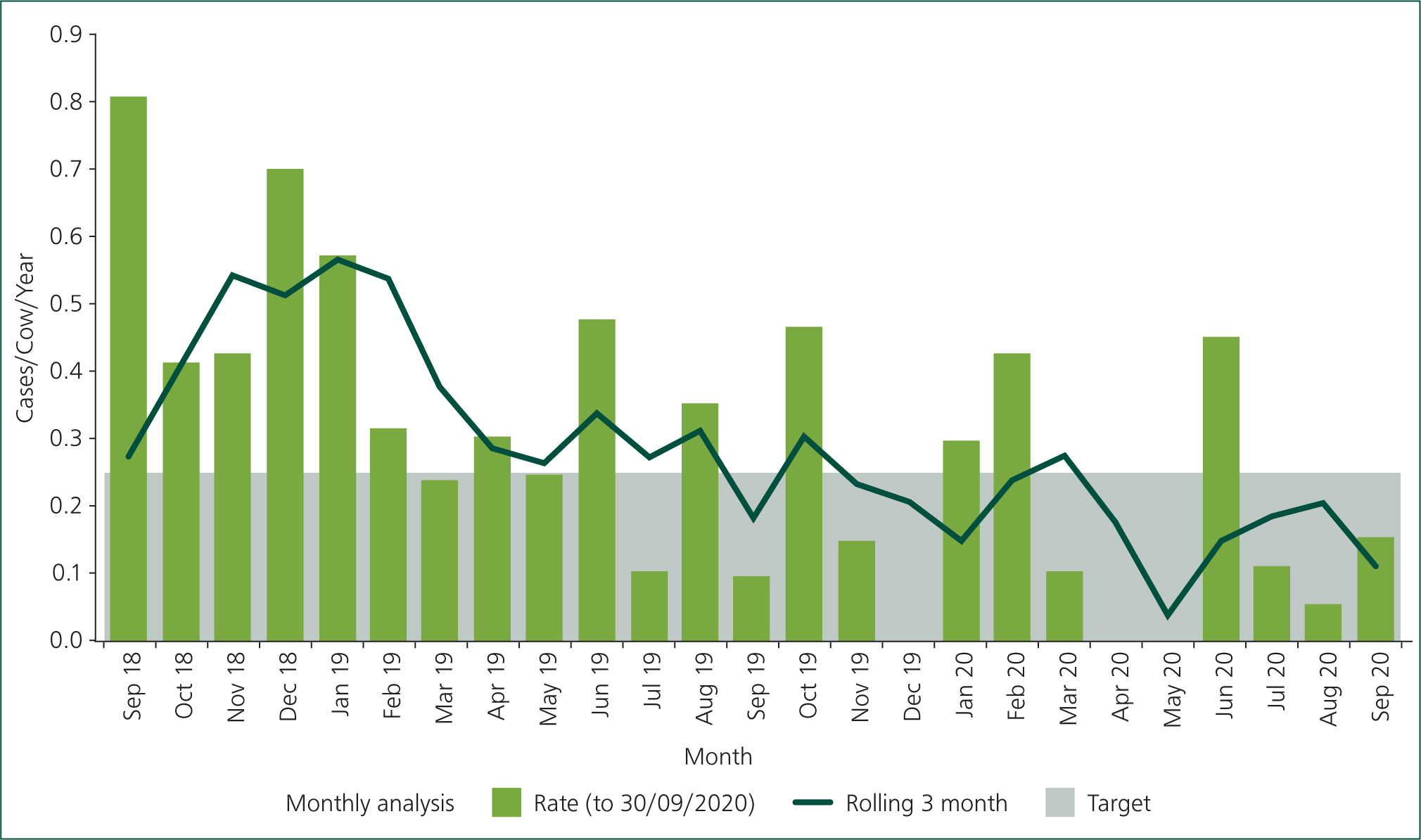
Whenever making changes in mastitis control, it is important to maintain recording of clinical cases: cases not treated should still be recorded. Figure 1 shows an example of how mastitis rate was apparently halved in a farm following implementation of OFC (June 2019). Through further discussion it was established that the farmer was only recording cases that were treated. One strategy for improving clinical mastitis recording is to give a non-antimicrobial treatment such as an anti-inflammatory or ‘uddermint’. This is also important in farms that may be withholding antimicrobial treatment in mild ‘grade I’ mastitis.
Zoonotic risk
Alongside farmer compliance, the potential zoonotic risk should also be considered. As far as the author is aware, no cases of food poisoning have been attributed to use of OFC plates, however there is the potential to culture bacteria that are capable of causing disease in humans. Bacterial species that can be found in mastitis and raw milk include S. aureus, Listeria monocytogenes, E. coli 0157, Campylobacter spp., Salmonella spp. and Bacillus cereus. As part of farmer training, clients should be made aware of the risk of opening plates, and disposing of culture media safely. OFC should be carried out in a biosecure area that is not used for food preparation or storage.
Novel test kits
This article has focused on commercial tests using semi-conventional bacteriological culture techniques. Readers should also be aware of some other approaches. A modified enzyme-linked immunosorbent assay (ELISA) test has recently been developed in New Zealand: it has high sensitivity and specificity for diagnosing S. uberis and S. aureus (Jones et al, 2019), and also gives an indication of antimicrobial sensitivity. In a pilot study, antimicrobial use was reduced by 24% with no significant difference in cure (Bates et al, 2020). Compared with conventional treatment with penicillin, the probability of S. aureus cure was numerically (but not statistically) higher, likely caused by detection of penicillin resistance and treatment with an alternative drug.
Another technique currently being researched is loop-mediated isothermal amplification (LAMP). Highly sensitive and specific assays for diagnosing certain mastitis pathogens have been described in the literature (Bosward et al, 2016; Cornelissen et al, 2016; Sheet et al, 2016), although at the time of writing no kits are commercially available.
While these new techniques have the potential for improved accuracy and treatment efficacy, at the cow level they are likely to be more expensive than OFC kits (typically around £5 per case).
Conclusion
OFC is one approach to reducing antimicrobial use in mastitis control, although from a purely financial perspective it is unlikely to be cost-effective on most UK farms. Several peer-reviewed studies have demonstrated a reduction in antimicrobial use, without ‘significant’ effect on clinical cure. Farms wishing to adopt OFC should consider the pathogen prevalence on their unit, and the risk of misclassifying Gram-positive species. Compliance with treatment protocols, health and safety guidelines and continued recording of clinical cases should all be discussed. Veterinary surgeons and advisors should also consider other approaches to mastitis control, specifically interventions to reduce new clinical cases. Those working in the UK should be directed towards excellent resources provided by the Agriculture and Horticulture Development Board (AHDB) and British Cattle Veterinary Association (BCVA) as part of the QuarterPRO initiative and the Mastitis Control Plan (Box 1).
Box 1.Useful information
- https://ahdb.org.uk/quarterpro
- https://www.bcva.org.uk/content/quarterpro-adviser-training
- https://ahdb.org.uk/mastitis-control-plan
- https://www.mastitiscontrolplan.co.uk/
KEY POINTS
- On-farm culture offers reasonable sensitivity and specificity in differentiating Gram-postivie and Gram-negative bacteria, but should not be used for pathogen specific diagnosis.
- More research is needed into the result of delayed treatment of Gram-positive pathogens, particularly in UK herds.
- More research is needed into the result of withholding treatment in non-E. coli Gram-negative species.
- Quality control, compliance with treatment protocols and recording of clinical cases should be discussed.
- On-farm culture and treatment will have no effect on new cases and new infections. In the short term it may lead to under-recording of clinical cases to the detriment of surveillance.
- Avoid using on farms with a high prevalence of Serratia or Klebsiella spp., e.g. farms using recycled manure solids bedding.

