Digital dermatitis is a common lesion causing lameness in dairy cattle (Barker, 2007), but also affects beef cattle (Sullivan et al, 2015) and sheep (Duncan et al, 2014). The average prevalence of digital dermatitis in dairy cattle can exceed 30% (Bell, 2006; Vink, 2006; Stokes, 2011), with a significant seasonality largely influenced by winter housing risks (Bell, 2006; Stokes, 2011). While many lesions do not cause clinical lameness (Stokes, 2011), volatility in herd mobility scores can often be attributed to repeated breakdowns in control of digital dermatitis (Bell and Main, 2012).
Digital dermatitis is a costly disease, leading to milk yield loss (Relun et al, 2013; Gomez et al, 2015; Kasiora et al, 2021) and reduced fertility (Gomez et al, 2015). While some cows with necrotic hoof lesions become chronically lame leading to culling, there are no reports of increased culling of cows with classic digital dermatitis lesions on the heels. Stochastic modelling would indicate the cost per case could be $64 USD (Dolecheck et al, 2019), although other studies have reported higher figures with UK conditions (Willshire and Bell, 2009).
Targeted treatment of cows with lesions, conducted hygienically to prevent further spread, is one logical component of control, along with prevention through environmental management (clean and dry feet), biocontainment (preventing spread to youngstock), biosecurity measures (closed herd) and foot cleaning and disinfection protocols (daily foot bathing). This paper examines the evidence for successful treatment of digital dermatitis.
Aetiology
Digital dermatitis is a polymicrobial infection, with many hundreds of bacterial species found in lesions (Krull et al, 2014; Zinicola et al, 2015). Changes in bacterial populations and relative abundance would support the treponemes as one of the major pathogens (Zinicola et al, 2015). Three phylotypes of treponeme (Treponema phagedenis, Treponema vicentii and Treponema pedis) are consistently found in lesions (Evans et al, 2008, 2009a), which has implicated these as primary pathogens. However, pure treponeme inoculates have proved only partially successful in reproducing disease in induction models, while full lesion homogenate material bandaged on abraded and/or macerated skin has been much more successful (Gomez et al, 2012; Krull et al, 2016a), suggesting an altered microbiome may be essential for lesion development. It also means antimicrobial therapy could target treponemes (narrow spectrum) or the many thousands of bacteria presented in the disrupted microbiome (broad spectrum), with the latter appearing more efficacious in practice.
Pathology
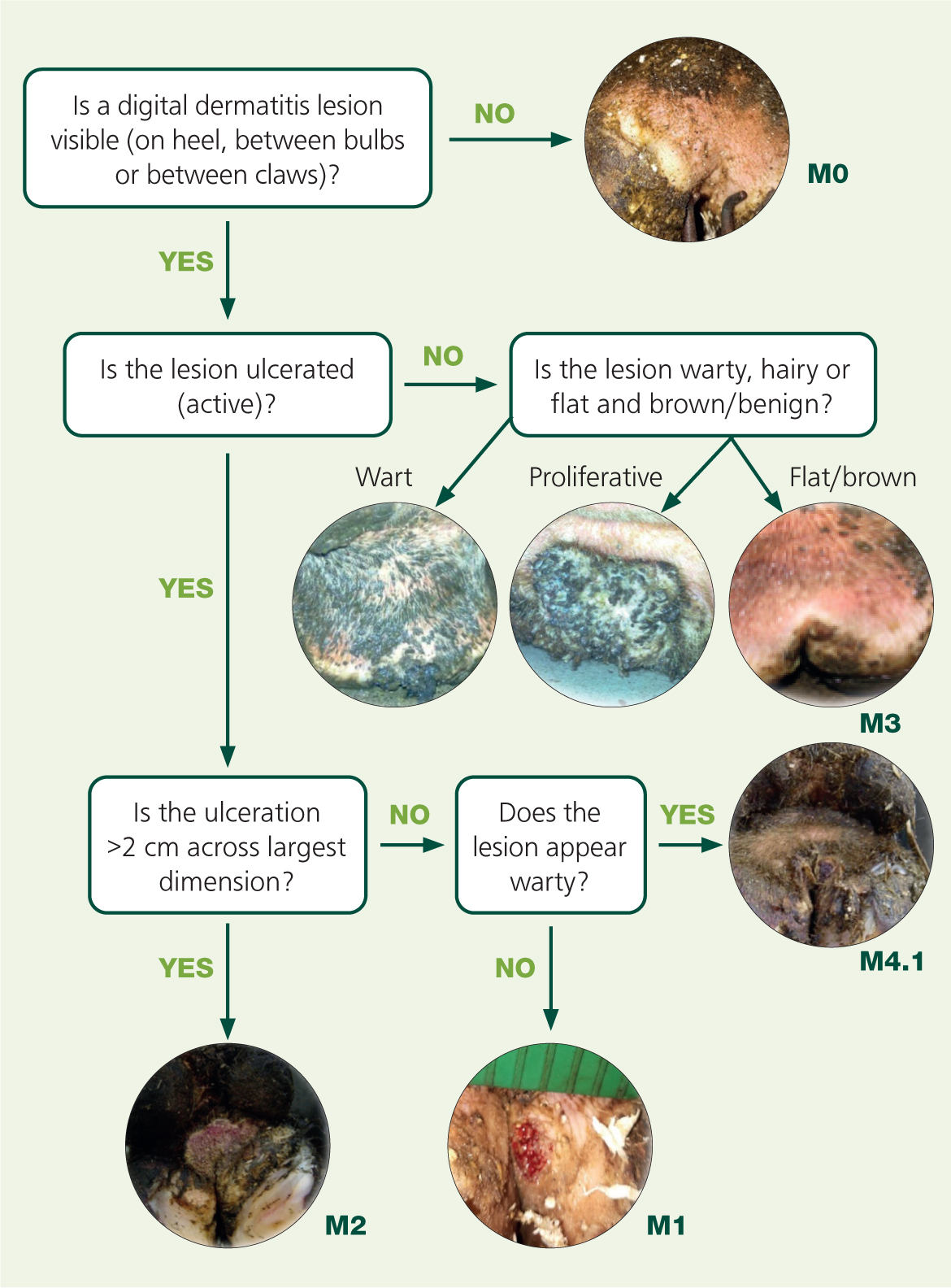
Digital dermatitis lesions range from superficial epidermal erosions and deep dermal ulcers to dyskeratotic/hyperkeratotic growths, and deeply inflamed ‘proliferative’ mixed skin lesions. The most commonly used grading system is the M-score (Dopfer et al, 1997; Berry et al, 2012; Kofler et al, 2020), with M1 and M2 representing erosive or ulcerative ‘active’ lesions, M3 healing lesions, M4 chronic hyperkeratotic lesions and M4.1 reactivating hyperkeratotic lesions. A simple flow chart can assist with scoring (Figure 1), which can be useful for screening cases for treatment and monitoring successful control. M-score can also be useful for deciding appropriate treatment. The M-score has just been applied to the digital dermatitis lesion of the foot, and so it is important to recognise that other digital dermatitis infections are present elsewhere on the animal too, such as hocks (Clegg et al, 2016a), hoof lesions (Evans et al, 2011) and treponemes are implicared in ischaemic teat necrosis (Clegg et al, 2016b), and ulcerative mammary dermatitis (Evans et al, 2010).
Reservoirs of infection and transmission
Treponemes are anaerobic spirochaetal bacteria that are difficult to isolate except in lesions, on foot trimming equipment and in cow foot prints (Sullivan et al, 2014; Bell, 2017). Lesions represent the major reservoir of infection, particularly the M4 lesions, which can reactivate within a few days. The slurry foot print and fomites are considered routes of transmission. Treponemes would appear to invade the skin through hair follicles and sebaceous glands (Evans et al, 2009b) or through wounds (Krull et al, 2016a). Under ideal induction conditions with inoculation of skin wounds, lesions can develop within 21 days (Gomez et al, 2012; Krull et al, 2016a), but the average time from initial skin pathology to clinical lesion can be 133 days (Krull et al, 2016b). These findings indicate there is good potential to prevent new infections with hygiene measures, minimised skin abrasions/maceration and with foot disinfection approaches, and if infection does become established, there may be opportunity to treat lesions prior to severe or progressed disease. The findings would also support the concept of treating all cows with visible or active digital dermatitis lesions (e.g. blitz treatment), to reduce infectious disease burden.
Note, bacteriological cure rates may be low, even when an apparent clinical cure is achieved (Berry et al, 2010). Therefore, treatment of lesions without other prevention strategies may have limited efficacy, particularly as many early lesions will be hidden between the heel bulbs and in the interdigital space, and reactivation of carrier animals can be a matter of a few days without other controls.
Given treponemes have been found in claw, hock and mammary skin lesions (Evans et al, 2010; Evans et al, 2011; Clegg et al, 2016a), these could represent a significant reservoir of infection in some herds and should therefore be included in treatment protocols. Necrotic hoof lesions can be successfully treated by deep and thorough surgical debridement under regional local anaesthesia (Bell and Mahendran, 2017), with case series reporting good success (Alcock, 2015; Kofler et al, 2017).
Topical antibiotic versus injectable antibiotic treatment
There is only one injectable antibiotic product licensed for digital dermatitis in the UK, which contains cefquinome, a fourth-generation cephalosporin and therefore a treatment of last resort (if at all). Other injectable antibiotics are licensed for general skin infections containing cefalexin and amoxicillin, and would be more appropriate, if used. While these treatments are available, with reports of complete response to injectable antibiotic treatment (Read and Walker, 1998), the hyperkeratotic and necrotic nature of most lesions means injectable antibiotics are unlikely to penetrate lesions sufficiently well alone. Cleaning, debridement and topical treatment appears more effective as demonstrated in one Canadian study (Silva et al, 2005). Given targeted topical treatments appear more efficacious, they use a lower quantity of antimicrobial, and carry less risk of resistance selection pressure, topical treatment represents the most responsible use of antibiotic for this condition, particularly given the high prevalence in herds.
The treponemes are sensitive to a range of antimicrobials (Evans et al, 2012). Antibiotics with the lowest minimum inhibitory concentrations (MIC) for treponemes include penicillin, erythromycin, ampicillin and oxytetracycline (Table 1). Of these only oxytetracycline is licensed for digital dermatitis specifically, but there are no published comparable data on MIC for other licensed antimicrobials (namely thiamphenicol, chlortetracycline and injectable cefquinome).
Table 1. Minimum inhibitory concentrations (MIC, mg/litre) for antimicrobials tested in vitro
| Penicillin | Erythromycin | Ampicillin | Oxytetracycline | Spectinomycin | Gentamicin | Lincomycin | Enrofloxacin | |
|---|---|---|---|---|---|---|---|---|
| Treponema vincintii | 0.003–0.375 | 0.003–0.375 | 0.0059–0.75 | 0.023–3 | 0.375–48 | 0.75–96 | 1.5–192 | 3–384 |
| Treponema phagedenis | 0.003–0.375 | 0.0059–0.75 | 0.0059–0.75 | 0.023–3 | 0.375–48 | 0.75–96 | 1.5–192 | 3–384 |
| Treponema pedis | 0.0059–0.75 | 0.0059–0.75 | 0.0117–1.5 | 0.0469–6 | 0.75–96 | 0.1875–24 | 0.375–48 | 3–384 |
(summarised from Evans et al, 2012)
MIC perhaps becomes less critical for topical treatments, provided there is sensivity, as relatively large concentrations are applied, generally repeatedly. For example, the standard oxytetracycline canister contains 4 g of oxytetracycline or approximately 200 mg per application, which far exceeds MIC for treponemes at the wound surface. In comparison, a US trial applied 2–25 g of oxytetracycline powder directly to lesions via paste or powder with bandage to represent the range of normal practice with administering antibiotic powders (Cramer et al, 2019). Alarmingly, this quantity and the methods of application led to detectable milk residues. Given the standard lactating cow intramammary tube contains around 200 mg antimicrobial, this study applied 10–100 times the anti-microbial content. Routine use of antibiotic powder in this way could massively inflate antimicrobial usage levels. Therefore, offlabel use of powder forms of oxytetracycline, tylosin, erythromycin or lincomycin can no longer be ethically justified for routine treatments, nor is it deemed necessary.
Single or repeated applications of tetracycline sprays appear highly efficacious for the treatment of digital dermatitis in most studies, which has been reviewed previously (Laven and Logue, 2006; Potterton et al, 2012). Repeated applications undoubtedly improve the outcome for more severe and persistent lesions, so marking affected limbs for re-treatment on a daily basis for 3 days (at least) would seem sensible, and was associated with higher cure rates in a review of research trials (Potterton et al. 2011). It is interesting to note definitions of lesion cure vary between studies, with some researchers accepting M4 (chronic, hyperkeratotic) as treatment success. Initial comparisons between thiamphenicol and oxytetracycline showed a statistically significant better cure rate for thiamphenicol with a 14% higher cure rate (89% CI 0.78 to 0.94 thiamphenicol versus 75% CI 0.67 to 0.86 oxytetracycline) in one Dutch study (Holzhauer et al, 2017).
Non-antibiotic alternatives
Minimising antimicrobial usage (AMU) in livestock is a national and global priority (O'Neill, 2015). Licensed topical antibiotic treatments are currently excluded from many calculations of dairy farm usage, in spite of being equivalent in AMU (mg/population corrected unit) to a lactating cow intramammary tube.
Currently there is only one non-antibiotic treatment licensed for digital dermatitis in the UK: Hoofit Gel (Intracare, The Netherlands). This product contains activated copper and zinc chelate. In trials it has performed as well as antibiotic controls (Holzhauer et al, 2011; Dotinga et al, 2017; Klawitter et al, 2019). Currently only the gel formulation is licensed in the UK, and not the more popular spray canister or knapsack spray.
Outside the UK, other non-antibiotic biocides are licensed for treatment. The most notable are the products containing salicylic acid, with or without other antibacterial or diffusing agents. Salicylic acid is commonly used in human skin products for warts and acne. Used topically it has antibacterial, antifungal, anti-inflammatory and keratolytic properties, which make it particularly suitable for hyperkeratotic digital dermatitis lesions. In one trial, 10 g of pure salicylic acid applied topically to digital dermatitis performed better than the antibiotic control (Schultz and Capion, 2013). Currently, salicylic acid is a licensed biocide in the UK, but not a licensed treatment for digital dermatitis. There are reasonable concerns that applying an acid to a lesion could be painful and deleterious to wound healing. However, low pH solutions (e.g. sodium bisulphate, organic acids) are frequently applied through footbaths to good effect and so this is a subject that requires further trial work to evaluate. Treponemes are quickly killed at pH under 6 (Bell, 2017), and salicylic acid has one of the lowest minimum bactericidal concentrations (MBC) in in vitro trials (Hartshorn et al, 2013), in addition to removing hyperkeratotic material.
Other licensed biocides have been tested in topical applications. Laven and Logue (2006) reviewed the evidence for iodine, copper sulphate, ionised copper, hydrogen peroxide, acidified sodium chlorite and glutaraldehyde. Only acidified sodium chlorite and one of the ionised (acidified) copper trials were as effective as the antibiotic control. Since that review, one study tested 1% hypochlorite with some success (Silva et al, 2005).
Non-steroidal anti-inflammatory drugs
Digital dermatitis is an infection that generates considerable inflammation (Blowey and Sharp, 1988), pain (Cutler et al, 2013) and causes lameness (Murray et al, 1996) with significant effects on milk yield (Kasiora et al, 2021) and fertility (Gomez et al, 2015). Ketoprofen has been shown to reduce the hyperalgesia associated with painful foot lesions, which included digital dermatitis, among others (Whay et al, 2005). In a recent randomised control trial, lame cows with digital dermatitis and treated with oxtetracycline had 20 times lower odds of being lame the next week if given ketoprofen compared with controls (Kasiora et al, 2021). Furthermore, if clinically lame and 1–16 days in milk, cows produced 10.5 litres more milk per day during the period of evaluation compared with controls. This fell to 5 litres more per day after the freshly calved period. Therefore, the use of pain and inflammation management is highly recommended as part of routine treatment of lame cows with digital dermatitis.
Other adjunctive therapies
There are a number of adjunctive therapies to consider when formulating treatment protocols. These include cleaning, drying and debriding lesions; daily disinfecting the foot at a following treatment (and prior to treatment); applying a bandage and other wound supporting applications.
Unless cows are out at clean pasture, digital dermatitis lesions will often be covered in slurry or mud together with scabs, fibrin and exudates. Thorough cleaning and then drying of the lesion is important to allow topical treatments to contact and penetrate the lesion. One study demonstrated improved cure rates when thorough debridement was conducted under intravenous regional anaesthesia (Silva et al, 2005).
When cleaning and debridement of lesions is performed, there is a high risk of hands and equipment becoming contaminated, as shown by researchers at the University of Liverpool (Gillespie et al, 2020). The Liverpool group showed 20 seconds of contact with 1% (v/v) iodophore disinfectant, 2% (w/v) Virkon® or 2% (v/v) sodium hypochlorite was sufficient to remove treponemes. This disinfection procedure clearly has to be balanced with time available, and so walking cows through foot baths after treatment could achieve a similar end result where daily footbathing is used on farm.
Despite strong opinion, the evidence-base on bandaging is weak with the balance in favour of bandaging. Currently there are three randomised control trials to consider.
A US randomised control trial evaluated treatment with bandage versus no bandage with treatment and reported rates of healing at 3–7 days and 8–12 days (Cutler et al, 2013). The percentage of healed lesions for the bandage versus no bandage was 42.9 versus. 22.2 at day 3–7, and 57.1 versus. 47.4 at day 8–12. The only statistically significant result was the comparison with treatments and negative control, and the difference between healing at day 3–7 versus day 8–12, indicating no benefit to bandaging. A post hoc power calculation showed the study needed 568 animals rather than 214 to determine if the observed effect size would be statistically significant. Furthermore, healing rates were relatively low as many lesions require 30 days for full wound healing following treatment, so rather equivocal evidence either way.
A German randomised control trial compared activated copper/zinc chelate versus chlortetracycline applied with or with a bandage (Klawitter et al, 2019). The bandage was a four-layered dressing consisting of gauze, cotton wool, non-elastic bandage and final layer of beech tar. Cows were re-examined every week and treatments reapplied until healed or until 4 weeks after treatment. The research team found the bandage was significantly more likely to result in cure (roughly double the cure rate which was defined as M0, i.e. no visible lesion) than no bandage, and there was no statistical difference between antibiotic and copper gel treatments. This is the strongest evidence for the benefit of bandaging although the bandage is not representative of common practice in the UK, rates of improvement looked good for the non-bandage treatments and no footbathing was conducted throughout the trial period.
Another randomised control trial looked at the use of polyurethane (PU) dressings with and without topical antibacterial treatments. The researcher evaluated three treatments: salicylic acid with 5-day bandage; oxytetracycline as a single application without a bandage; and a PU bandage applied for 10 days without topical antibacterial agent (Fiedler et al, 2015). Lesions were followed to 36 days after treatment and there was no significant difference in cure rates between the three groups. While this did not show a benefit to bandaging per se over the other treatments, all three treatments were significantly better than negative controls at day 10, showing the bandage alone could be as important for healing as the topical treatment. The authors comment on bandage injuries occurring in two cows, which is perhaps one of the clearest findings against bandage use.
It has been noted that typical bandages become wet within 2 days of application under standard UK conditions involving pasture-access and daily foot bathing (Mahendran et al, 2019). Wet conditions are likely to macerate skin, delay wound healing and predispose to new infections. Furthermore, they are often over-looked when it comes to removal, with the risk of cutting into the skin and creating ischaemic injuries to the distal limb. Conse-quently, many experts advocate no bandage, some advocate ‘bikini wraps’, which are very light dressings which stay on (or fall off) after 8–12 hours, while others advocate a thick bandage with barriers against moisture ingress. A novel type of bandage is now available that dissolves in contact with water, removing some risk, but shortening the bandage treatment. Needless-to-say, further work is needed to establish if there is an optimal bandage technique, and if it is beneficial under UK conditions. Interestingly, the author has observed that healing rates appear very good when blocks are applied to elevate the lesion out of slurry and this could be an alternative (albeit extreme and costly) approach to keep healing wounds clean and out of slurry. As a minimum we should be ambivalent towards bandaging as part of digital dermatitis treatment, and at least acknowledging if managed well, bandages can enhance recovery from digital dermatitis in some dairy environments. More research is required to explore the relative benefits of bandaging compared with repeated (daily) topical treatments. It is important to consider the ethics of spray antibiotics, a practice now considered irresponsible in most parts of the world.
Foot bathing in the days following treatment is likely to be beneficial for healing. Laven and Hunt (2002) showed foot bathing for 7 days with 6% formalin, 2% copper sulphate or 1% peracetic acid promoted lesion recovery (at 21 days) without individual topical treatment. However, it is important to state the 3-week cure rates were low (45–75%), compared with >80% with conventional topical treatments in a crush. This study also showed the antibiotic footbathing did not perform any better than the disinfectant footbaths (50% cure rate at 21 days).
There are additional topical treatments that could enhance healing. One study looked at the use of topical vitamin D and concluded it helped (Watts et al, 2020). Following initial anti-biotic treatment, some clinicians advocate the use of ionised copper sprays to clean the lesion once or twice daily. Addressing macro- and micro-mineral status and biotin levels (at herd level or by using a bolus for individual animals) could improve rates of lesion keratinisation as seen in sole ulcers (Bergsten et al, 2003), particularly in situations of deficiency or high milk yield, but little research has been done looking specifically at digital dermatitis recovery.
Herd treatments
This article has mainly focused on the treatment at the individual animal level. As mentioned at the start, the main reservoir of infection are the animals with lesions. Treating all affected animals all at once can reduce overall herd-level infection pressure and has been called the ‘blitz treatment’ (Pedersen, 2019). This approach involves screening cows in the parlour with a hose and bright light (Rodriguez-Lainz et al, 1998), and noting cows with active lesions for treatment (particularly M2s). An alternative approach is to treat the whole herd through the crush at one time, particularly if combined with a general foot check and trim as necessary at the same time.
The blitz treatment and whole herd trim can be labour intensive and costly, particularly if follow-up treatments are required for several days. Some elements of follow-up treatment can be performed in the parlour, but these are best reserved for lesions that have been cleaned and debrided in the crush. It is now permissible under UK Red Tractor rules to use topical antibiotic spray treatments in the parlour at milking, but disinfectant biocides could be preferable in the parlour.
In situations with a high prevalence of digital dermatitis, it may be more efficient to run all but the more severely affected cows in the herd through an acidified (ionised) copper sulphate footbath for 3 days and then conduct the digital dermatitis screen 7–10 days later. It is advisable to monitor cows for signs of discomfort and if cows are unfamiliar with routine bathing to then warn the producer that cow flow through the footbath may be slow initially as cows get used to going through the bath for the first time. Copper is not an EU approved biocide but can be permitted if used at low concentrations (below 1%). The MIC of copper sulphate is <0.01% in the absence of manure (e.g. knapsack spray) and <0.3% in the presence of 20% manure contamination, so ionised solutions at 1% should prove effective and expect similar results to those reported by Laven and Hunt (2002). Footbathing with a suitable biocide should be continued on a daily basis after treatments are complete (but avoid bathing on days cows are treated to avoid washing off treatments). Opinion has gained greater consensus on many aspects of foot bath design (Cook et al, 2012), foot bath biocide use (Bell et al, 2014) and general management of digital dermatitis (Bell and Vanhoudt, 2020), and readers are encouraged to consider these in combination with proactive treatment strategies.
Conclusion – the recommended treatment protocols
Prevention is the ideal means of controlling digital dermatitis. However, the major reservoirs of infection are the infected foot lesions, so the author advocates treatment of all lesions regardless of severity or M-score, ideally all at once, and including necrotic hoof lesions like toe necrosis. In most instances on-farm treatment of digital dermatitis is kept very simple and generic, with only cows showing severe active lesions receiving treatment. However, there is a strong rationale to minimise antibiotic usage and so non-anti-biotic alternatives can be adopted for all but the more severe or painful active lesions (i.e. M2 lesions, not the less painful and more chronic M4 and 4.1 lesions). Given the inflammatory and painful nature of the disease, NSAIDs are recommended for mobility score 2 and 3 cows with painful digital dermatitis lesions. Finally, if used, bandages must be combined with protocols to ensure their correct application and prompt removal at the appropriate time. Table 2 summarises an approach to treatment that can be adapted or used under veterinary instruction following staff training.
Table 2. Gold standard digital dermatitis treatment protocol based on published evidence (to be delivered through veterinary advice)
|
|
|
|
|
|
|
|
|
|
|
|
|
KEY POINTS
- Digital dermatitis is one of the commonest lesions causing pain and lameness in dairy cows, with reports commonly describing over 30% of cows with lesions at one time.
- Control generally involves good biosecurity with biocontainment of infected groups or individuals, underfoot hygiene measures, foot disinfection and treatment of cows with lesions.
- Treatment of all active lesions at once improves welfare, herd mobility and reduces disease infection pressure.
- Digital dermatitis is a polymicrobial infection of the skin with three phylotypes of treponeme repeatedly found in lesions and not healthy skin.
- Digital dermatitis is highly responsive to antimicrobial treatments containing tetracyclines or thiamphenicol combined with cleaning, debridement and licensed non-steroidal anti-inflammatory drugs (ketoprofen).
- Non-antibiotic alternative treatments appear equally efficacious, and apart from copper gel, are unlicensed, invariably require bandaging and optimal bandage technique/protocol is still unclear.


