Digital dermatitis (DD) is a multifactorial disease, both in terms of the pathogens that can inhabit lesions, and in terms of the environmental and management conditions which are presumed to cause damage to the stratum corneum and changes to the foot-skin microbiome, thus allowing pathogens to cause lesion development. Gaps in our understanding of the complex pathophysiology of DD alongside practical problems with implementing control measures are hindering our efforts to eliminate disease.
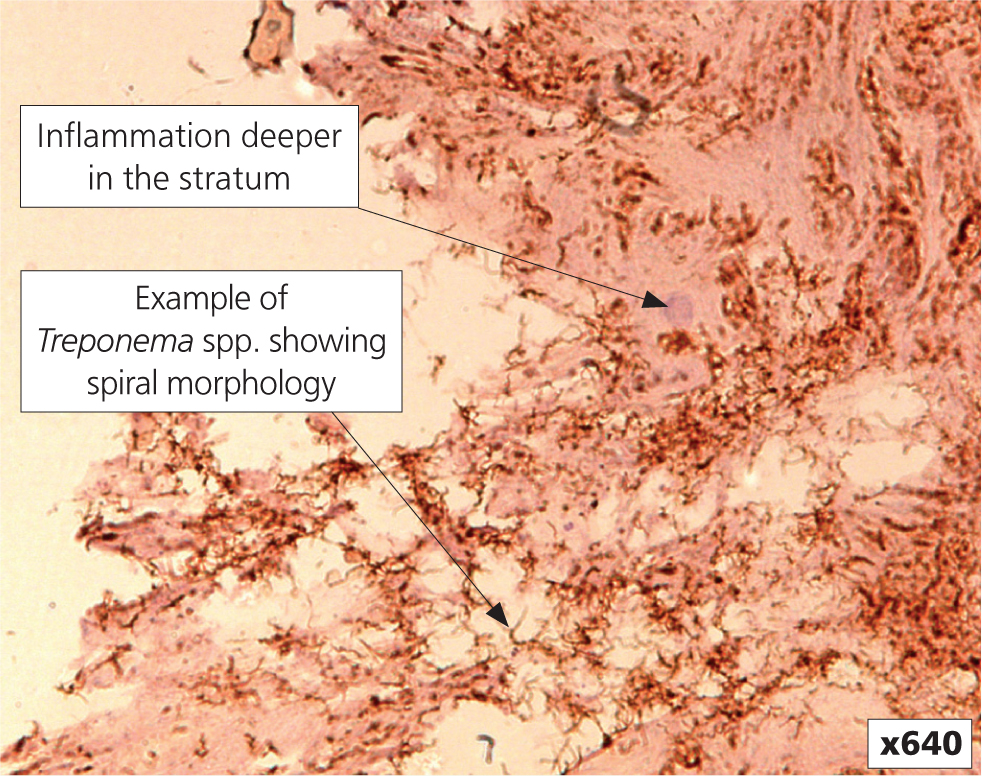
A wide variety of bacteria have been detected from DD lesions, including Fusobacterium spp., Bacteroides spp., Guggenheimella bovis, Campylobacter spp. and Peptococcus spp; and more recently Porphyromonas levii, Mycoplasma spp. and Prevotella spp. (Berry et al, 2010). However, it is currently understood that there are specific Treponema phylogroups that are important in causing DD and they are found in combination (Evans et al, 2016). More detailed genotypic and phenotypic characterisation carried out on UK isolates demonstrated three distinct taxonomic groups, which were designated as Treponema medium/vincentii-like, Treponema phagedenis-like, and Treponema putidum/denticola-like (Evans et al, 2008). More recently the latter phylogroup has been re-classified as Treponema pedis (Evans et al, 2009); further taxonomic scrutiny of the other two groups is still needed to distinguish them from the human treponemes they were found to be similar to. Only treponemes have been found deep in the epidermis (Figure 1), with tissue invasion considered a major virulence trait (Blowey et al, 1994; Dopfer et al, 1997; Moter et al, 1998). The importance of treponemes in DD is supported by successful development of infection models (Read and Walker, 1996; Gomez et al, 2012; Krull et al, 2016), however skin needs to be macerated to allow infection to become established; and the use of tissue homogenate prepared from fresh lesion material is more effective than inoculation with pure Treponema spp. cultures.
Infection reservoirs
The most recently calculated reproduction ratio (R0) for DD is 2.36, meaning that in the absence of control measures it is expected that each case will give rise to 2.36 further cases (Biemans et al, 2018). When R0 is reduced below one the number of new cases will reduce, and the lower the value, the more likely it is that eradication can be achieved. To prevent spread of DD, infection reservoirs for the pathogenic Treponema phylogroups need to be identified and controlled.
The lesions
It is widely considered that the most important source of infection are the lesions themselves, particularly M4 lesions (Figure 2). The lesion scoring system proposed by Dopfer et al in 1997 and adapted by Berry et al in 2012 describes the development of disease from small focal active lesions (M1), through the larger ulcerative active stage (M2), then on to a healing stage (M3), and the development of a chronic lesion with a hyperkeratotic scab which is still considered infectious (M4) (Figure 2). Treponemes have an encysted form that is suspected of lying dormant in lesions deep in bovine skin, contributing to the chronic form of the disease (Dopfer et al, 2012a). For reasons not fully understood, M4 lesions can ‘reactivate’ giving rise to chronic lesions with new focal active lesions superimposed (M4.1). Although there is little difference in transmission rate depending on lesion class, as 70% of the infectious time is spent at M4, this class contributes 88.5% to R0 (Biemans et al, 2018). Prevention of progression of active lesions to the M4 form is crucial to reducing cases.

Broadly, our options are individual or collective treatments (footbathing). Early individual treatment is important for promoting healing from M2, while effective footbathing slows the transition from M4 back to M2 (Dopfer et al, 2012b). Current industry advice for individual treatment is to use licensed topical oxytetracycline or thiamphenicol spray (Holzhauer et al, 2017), however it is possible that non-antibiotic alternatives have similar efficacy (Manning, 2016). For footbathing, there is little evidence to prompt recommendation of one product in favour of another. The most recent review of published footbathing trials found only 5% copper sulphate used at least four times per week could be superior both to no footbath and to water only (Jacobs et al, 2019). It is certainly no longer acceptable to use antibiotics in this fashion (Bell et al, 2017) due to mass exposure of animals to products which may lead to an increase in antimicrobial resistance, and also soil exposure to the same selection pressure if slurry (which typically includes footbath waste) is spread on agricultural land.
Industry recommendations advocate the use of footbaths 3–3.7 metres long and 0.5–0.6 metres wide to ensure all four feet are submerged twice, and at least 12 cm deep to ensure the whole hoof is covered by solution to the coronary band, even at the cranial aspect of the hoof (Cook et al, 2012). Footbath volume and disinfectant used should be measured each time to ensure correct concentrations and contact times are achieved. Footbath contamination levels resulting in product inactivation is largely unknown, although EU Regulation 528/2012 specifies biocides have to be effective against Enterococcus hirae, Proteus vulgaris, Pseudomonas aeruginosa and Staphylococcus aureus at contamination levels of 20 g/litre organic matter (Ariza et al, 2019). Laboratory testing of a range of disinfectants showed that minimum bactericidal concentrations for a Treponema phagedenis-like isolate in the presence of 20 g/litre organic matter were below recommended working concentrations (Hartshorn et al, 2013). Footbaths frequently become contaminated above this level, and footbaths often deplete below the recommended depth, leading to poor foot coverage (Ariza et al, 2019). The use of automatic footbaths may solve these practical problems with minimal time investment, however their benefits for reducing DD incidence have not been proven in peer-reviewed literature.
The environment
Environment muddiness has been identified as a risk for DD (Rodriguez-Lainz et al, 1996). Furthermore good animal hygiene reduces the risk of DD (Hultgren and Bergsten, 2001) while increases in DD cases during housing under conditions of poor hygiene suggest faeces as a source of treponemes (Blowey and Sharp, 1988). Interestingly, detecting treponemes from environmental slurry samples and individual fresh faecal samples using polymerase chain reaction (PCR) assays was unsuccessful (Evans et al, 2012). Metagenomic studies of slurry, however, did identify small numbers of Treponema spp. in DD-infected herds and their absence in healthy herds, suggesting that slurry may be a vehicle for spread, but not the primary infection reservoir (Klitgaard et al, 2017).
The gastrointestinal tract
Sampling of 44 sites from six cows from a DD endemic herd (three healthy animals and three affected by DD) to investigate the bovine gastrointestinal tract (GIT) as an infection reservoir using PCR found T. phagedenis-like DD spirochaetes in one DD-affected cow in oral gingival tissue, the rumen dorsal sac and the reticular pillar. The recto-anal junction from a healthy cow was also positive for T. medium-like DD spirochaetes. Further testing of oral gingival samples found 1/8 positive for T. pedis, and additional testing of recto-anal junction samples found DD spirochaetes in 4/21 samples (Evans et al, 2012). In a more recent metagenomic study Treponema spp. found in DD lesions were found in rumen and faecal microbiomes of cattle from DD infected farms (Zinicola et al, 2015). The importance of GIT carriage of treponemes in terms of transmission dynamics between and within farms is unknown, and more work is required to clarify the involvement of this infection reservoir in disease transmission.
The role of foot trimming in transmission
An epidemiological study published in 1999 associated the use of a primary hoof trimmer who trims cows' hooves at other farms, and lack of washing of hoof trimming equipment between cows being trimmed, with increased incidence (>5%) of DD in herds (Wells et al, 1999). A 2018 study of pasture-based herds in New Zealand supported these findings, and the authors concluded that farms with DD should ensure that hoof trimming equipment is disinfected effectively between cattle (Yang et al, 2018). DD treponeme DNA was detected on 17/17 hoof knives following foot trimming of clinical DD cases, and 7/8 gloves worn by a foot trimmer to trim feet of DD positive cows (Blowey et al, 2013). In addition an isolate belonging to the T. phagedenis-like spirochaetes was cultivated from a knife after trimming a DD positive cow (Sullivan et al, 2014) and they can be isolated in culture for up to 3 days from gloves contaminated during handling of sheep feet affected by the analogous disease contagious ovine DD (Angell et al, 2017). Over-all these data suggest hoof knives and gloves to be major control points for preventing transmission. It has also been shown that DD treponemes are culturable for at least 2 hours after application to hoof knife blades implicating hoof knives as fomites for carrying treponemes between cows during foot trimming (Gillespie et al, 2019). An evidence-based disinfection protocol has been developed with Agriculture and Horticulture Development Board (AHDB) Dairy to mitigate the risk of DD transmission during foot trimming (Gillespie et al, 2019). The protocol is available from the AHDB Knowledge Library at https://ahdb.org.uk/reducing-spread-of-DD.
Several potential infection reservoirs for DD have been identified, but the relative importance of each remains unknown. DD infection models have shown that for lesions to develop, existing tissue damage is required plus direct contact with a fresh lesional material containing viable polytreponemal bacterial load (Read and Walker, 1996; Gomez et al, 2012; Krull et al, 2016). The existing paradigm is that infection is spread from M2 and M4 lesions via the environment, however treponemes have not been isolated in culture from environmental sources or detected by PCR (Evans et al, 2012). They have been detected in slurry, the rumen and faeces by metagenomic analyses (Krull et al, 2014; Zinicola et al, 2015a; Zinicola et al, 2015b; Klitgaard et al, 2017), however sequencing methods are capable of detecting very small numbers of organisms, are less specific, and give no indication of viability and true ability to transmit and cause disease. Hence questions remain about the importance of slurry and the environment in transmission. The epidemiological association of poor environmental hygiene with increased DD prevalence could be due mainly to the detrimental effect on the condition of the foot skin. Table 1 rationalises the possibilities and makes the argument that foot-trimming is the only event during which it is known that viable treponemes contact the feet of other cows in the herd (assuming that effective disinfection is not practised). It is also more likely that there will be existing tissue damage, at least for lame cows being treated, that could make them more susceptible to acquiring the pathogens needed to cause DD lesions during foot-trimming, fulfilling the infection model criteria.
Table 1. Identified sources of infection on farm, fulfilment of infection model criteria and associated risk factors and microbiological evidence
| Source of Infection | Perceived likelihood of direct contact | Viable bacterial load | Associated risk factor evidence | Tissue damage / Infection reservoir evidence |
|---|---|---|---|---|
| Foot-trimming tools | HIGH | YES | Not washing hoof trimming tools in between animals=increased DD risk (Wells et al, 1999).Use of hoof trimmer who works on multiple farms= increased risk of DD (Wells et al, 1999; Yang et al, 2018) | Tools will make direct contact with damaged feet. DD is highly contagious The DD treponemes are identified frequently on hoof trimming equipment and can be cultured and can survive for up to 2 hours (Gillespie et al, 2019; Sullivan et al, 2014) |
| Foot trimming hands/gloves | HIGH | YES | Use of hoof trimmer who works on multiple farms= increased risk of DD (Wells et al, 1999; Yang et al, 2018) | Hands/gloves will make direct contact with damaged feet. DD is highly contagious. The DD treponemes are identified frequently on hands/gloves and can be cultured (Blowey et al, 2013; Angell et al, 2017) |
| Farm slurry | HIGH | ? | Increased environment slurry= in-creased DD risk (Rodriguez-Lainz et al, 1996).Increased hygiene = reduced DD risk (Hultgren et al, 2001) | Exposure of skin to moisture (such as moist slurry environment) needed for DD transmission (Read et al, 1996; Gomez et al, 2012; Krull et al, 2016). DD treponemes in oral and rectal cavities suggest faecal shedding (Evans et al, 2012), although cultivable treponemes not isolated from slurry |
| Oral or rectal cavity | LOW | YES | Increased hygiene = reduced DD risk | DD treponemes in oral and rectal cavities (Evans et al, 2012) and can be cultured from ruminant rectal tissue (Sullivan et al, 2015) |
| Lesions | LOW | YES | Mathematical model shows M4 to be the most significant lesion stage for transmission (Biemans et al, 2018) | DD treponemes culturable from lesions (Evans et al, 2008) |
Biosecurity
With infection reservoirs in mind, biosecurity measures must be considered both between herds, and within herds. Bringing infected animals to a farm is an important route of introduction (Rodriguez-Lainz et al, 1996; Wells et al, 1999) and must be avoided. Where buying in cattle from such a source is unavoidable, it is recommended to inspect all four feet carefully before animals contact the rest of the herd. Even so, because histological changes can occur in the foot-skin before morphological changes become apparent (Rasmussen et al, 2012), the risk from this route cannot be ruled out. Furthermore, now that the gastrointestinal tract has also been identified as a possible infection reservoir, it is possible that any cow brought from a DD-infected herd could carry the disease (Evans et al, 2012).
Within herds, cases can be decreased by reducing infection pressure. This can be achieved by early treatment of active M2 lesions and preventing transition of M4 lesions to M2 using effective footbathing (Dopfer et al, 2012b). Practising good environmental hygiene will decrease any potential for slurry and walking surfaces to act as infection reservoirs and cause damage to foot skin. Disinfection of foot-trimming equipment, particularly hands and hoof knives, would also serve to reduce infection pressure. Finally, treatment and prevention of DD in youngstock must not be over-looked as heifers that are affected prior to parturition are a source of infection to the main herd (Capion et al, 2009).
The future
Recent advances in metagenomic sequencing have made it possible to study the entire bacterial population of DD lesions, which has given insights into how these change in relation to lesion morphology (Krull et al, 2014; Zinicola et al, 2015a; Zinicola et al, 2015b). Studying molecular interactions between bacteria residing in lesions may reveal important pathological processes that cause transitions between lesion stages. Such systems approaches to understanding DD pathobiology holds potential for development of novel treatments which could be key to eradication (Orsel et al, 2018).
It has been confirmed that there is a genetic component to DD susceptibility (Scholey et al, 2012; Schopke et al, 2015). Quantitative trait loci associated with susceptibility to claw-horn lesions have been identified (Sanchez-Molano et al, 2019), and similar associations likely exist for susceptibility to DD. Recent work has shown that reducing DD prevalence by selective breeding is a promising strategy due to substantial heritable variation in susceptibility (Biemans et al, 2019a,b).
Conclusion
Recent research has improved our understanding of infection reservoirs for DD in the dairy herd and highlighted some of the challenges with eliminating disease As new avenues towards achieving eradication are explored, we need to continue to work with farmers to apply existing recommendations on farm. In most herds substantial improvements could be made by addressing practical problems such as increasing environmental hygiene, ensuring correct footbath use, and improving hygiene during foot trimming.
KEY POINTS
- A wide variety of bacteria are identified in digital dermatitis (DD) lesions however only Treponema spp. are consistently implicated in DD aetiology.
- Infection models have demonstrated that tissue damage is required for lesions to develop, as well as direct contact with fresh lesional material containing treponemes.
- As well as the lesions themselves, the environment and the gastrointestinal tract have been investigated as potential infection reservoirs.
- Foot trimming provides an opportunity for fresh lesional material containing viable treponemes to be transferred between cows' feet, frequently offering these pathogens damaged tissue to colonise.
- Good biosecurity is essential to prevent entry of DD into uninfected herds. It is recommended to inspect feet for lesions before bought in animals contact the rest of the herd. Due to possible gastrointestinal carriage of treponemes, however, absence of DD lesions does not guarantee animals from endemic herds are not carrying causative bacteria.
- Improving our understanding of DD, including both pathobiology and host genetic resistance, should open options for novel prevention and treatment strategies. In the meantime, we must continue to apply practical solutions such as enhancing foot-trimming hygiene and reducing the slurry exposure of animals together with appropriate, regular footbathing.


