The mortality and morbidity of neonatal lambs is a cause of poor welfare for affected cases and results in the reduced production and economic profitability of farms. Worldwide, on average, 15% of lambs die in the neonatal period, with this rate remaining unchanged for the past 40 years (Dwyer et al, 2016). After birth, the neonate enters a new and hostile environment compared with that in utero and in particular must meet the challenge presented by infectious organisms which rapidly become important causes of mortality for neonatal lambs.
In recent surveys, infectious disease accounted for 10% of neonatal deaths in Wales, UK (Hybu Cig Cymru – Meat Promotion Wales, 2011) and 36% in Norway (Holmoy et al, 2017), with both surveys reporting the majority of deaths within 48 hours of birth. Arguably, the most important infectious disease syndrome reported in this very early period is an endotoxaemia associated with undefined pathotypes of Escherichia coli, colloquially known as watery mouth disease (WM) on account of one of the clinical signs seen — drooling saliva from the mouth.
Although WM has been recognised since at least the 1920s (Eales, 1987), surveys have suggested that in intensively farmed sheep it can account for of up to a quarter of all neonatal deaths (King and Hodgson, 1991; Scott, 2007). Furthermore, WM is cited as the biggest reason for the widespread prophylactic and metaphylactic use of antibiotics in large numbers of flocks in some regions (King and Hodgson, 1991; Davies et al, 2017). This practice is clearly untenable, especially in light of the integrated global challenges of improving food security, maintaining and improving animal welfare and reducing the use of antimicrobials in farmed species (World Health Organization, 2015; O'Neill, 2016). For too long, WM has been neglected from a research perspective, and alternatives to mass antibiosis are urgently required. The aim of this review is to summarise the current knowledge of WM in lambs and clarify the evidence base for each aspect considered.
Materials and methods
Due to the small numbers of research papers published on WM, a broad, systematic approach was used to try to identify as many peripheral studies, letters and reports as possible (Figure 1). Three English language databases were used: PubMed, Web of Science and Scopus, from 1980–2019 inclusive. These databases were searched using predefined search terms (Table 1). The citations were primarily screened for eligibility by title and abstract, and the full text used for any ambiguous citations. Citations were excluded for the following reasons:
- If duplicated
- If not in English and a suitable translation was unavailable
- If considered irrelevant in terms of the disease process or body system considered; for example, if the study considered only the respiratory system
- If solely considered pre-term lambs or lambs older than 7 days
- If primarily investigating the LamB protein in E. coli.
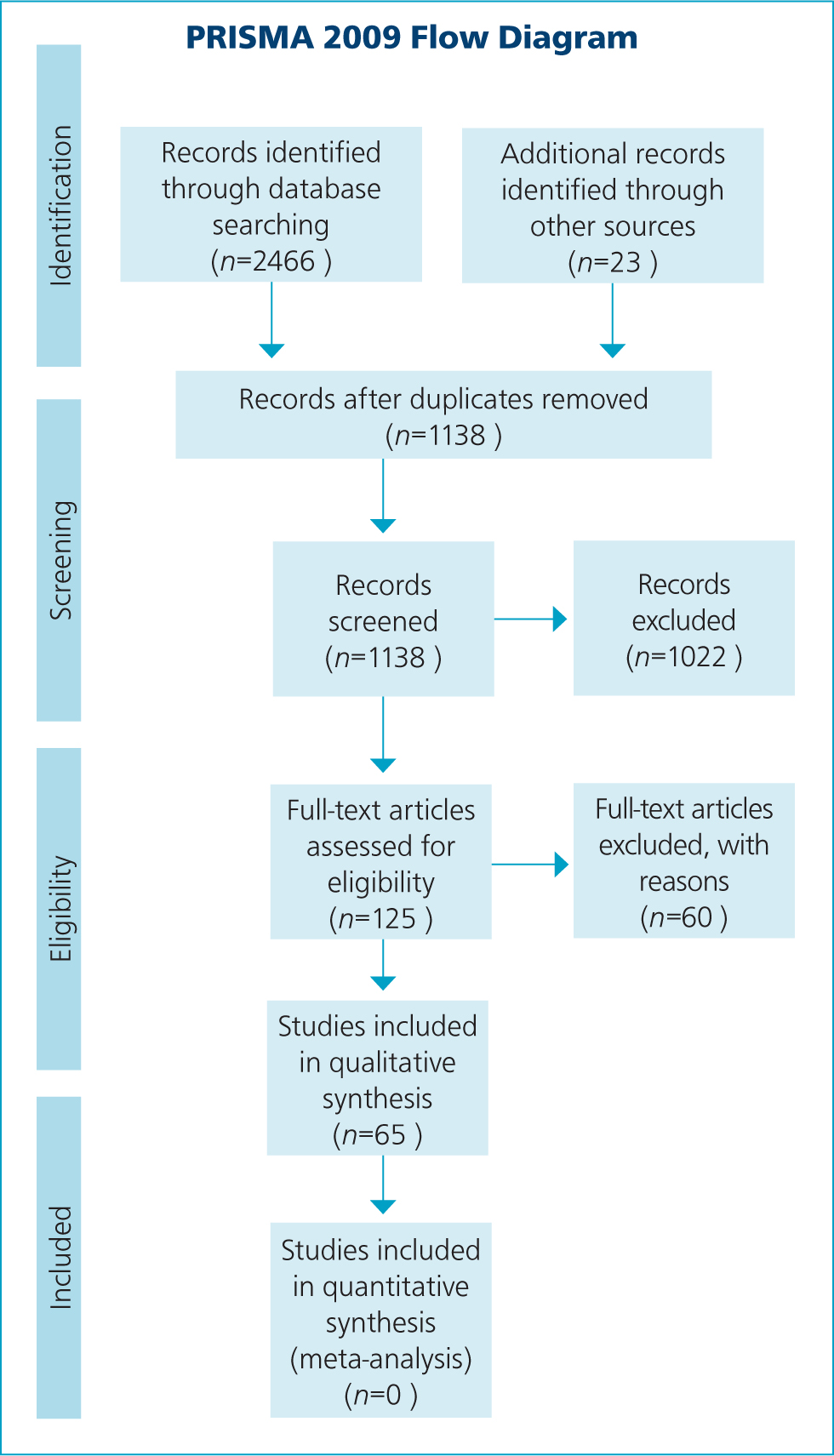
Table 1. Databases and search terms used in the systematic review process, together with the number of citations identified from each individual search
| Database and search terms used | N citations |
|---|---|
| PubMed – 29/11/2019 | |
| ‘watery mouth’[Title/Abstract] | 14 |
| (endotoxic[Title/Abstract] AND lambs[Title/Abstract] | 2 |
| (endotoxaemia[Title/Abstract]) AND lambs[Title/Abstract] | 5 |
| (endotoxin[Title/Abstract]) AND lambs[Title/Abstract] | 66 |
| (((Escherichia coli[Title/Abstract]) OR E. coli[Title/Abstract])) AND Lambs[Title/Abstract] | 201 |
| Web of Science – 29/11/2019 | |
| (TS=’watery mouth’) AND LANGUAGE: (English)) | 21 |
| (TS=(endotoxic AND lambs) AND LANGUAGE: (English)) | 6 |
| (TS=(endotoxaemia AND lambs) AND LANGUAGE: (English)) | 8 |
| (TS=(endotoxin AND lambs) AND LANGUAGE: (English)) | 209 |
| (TS=((Escherichia coli OR E. coli) AND lambs)) AND LANGUAGE: (English)) | 839 |
| Scopus – 29/11/2019 | |
| TITLE-ABS-KEY (‘watery mouth’) | 20 |
| TITLE-ABS-KEY (endotoxic AND lambs) | 4 |
| TITLE-ABS-KEY (endotoxaemia AND lambs) | 6 |
| TITLE-ABS-KEY (endotoxin AND lambs) | 95 |
| TITLE-ABS-KEY ((Escherichia coli OR E. coli) AND lambs) | 970 |
Following the initial screening process, a further 60 full-text articles were examined and removed. Throughout, a small number of additional articles (n=23) not identified through the original database searches were found by searching the reference lists of each citation and included if considered relevant.
Results
Clinical features
Twenty-four citations were obtained that described clinical features of WM (Table 2) and, of these, 15 specified a case definition. Increased salivation/mucus around the mouth was the only feature described by all 24 publications; 17 described cases as having reduced appetite or ceasing to suck; 17 described cases as having a distended abdomen/abdominal tympany; and 15 described cases as dull or depressed. Other features were less frequently reported, although in some citations were considered as key features of the disease, such as retained meconium or reduced gut motility (Mitchell and Linklater, 1983). In summary, the disease usually manifests in lambs <72 hours of age, presenting as dull/depressed with reduced sucking/inappetence followed by the development of increased salivation/mucus around the mouth — hence the name ‘watery mouth’ (Figures 2a and b). This feature has also led to the synonymous colloquialisms ‘slavers/slavery mouth’ (King, 1986; King and Hodgson, 1991), with two French reviews using the colloquialisms ‘agneaux baveurs’ (slobbery lambs) and ‘bouche baveuse’ (slobbery mouth) (Millemann et al, 2003; Poncelet, 2004).
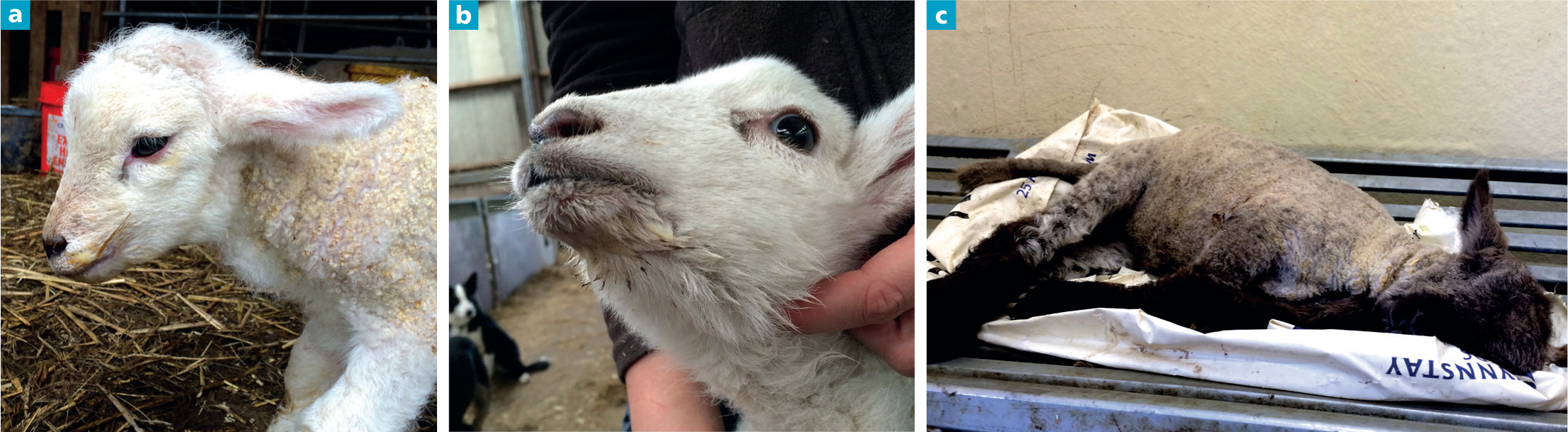
Table 2. Clinical signs of cases with watery mouth, as described or reported by publications, together with those that described the clinical feature as part of a specific case definition
Abdominal tympany appears later on in the disease process (Figure 2c) and several authors describe gentle shaking of the lambs eliciting a splashing sound, with a further colloquialisation of ‘rattle belly’ (Haig, 1981; King, 1986; Eales, 1987). Scouring is unusual and often constipation or retained meconium may be present (Eales et al, 1986). Untreated cases die within 6 to 24 hours showing a terminal hypoglycaemia, hypothermia and lacticacidaemia (Collins et al, 1985; Hodgson et al, 1989b).
Differential diagnoses
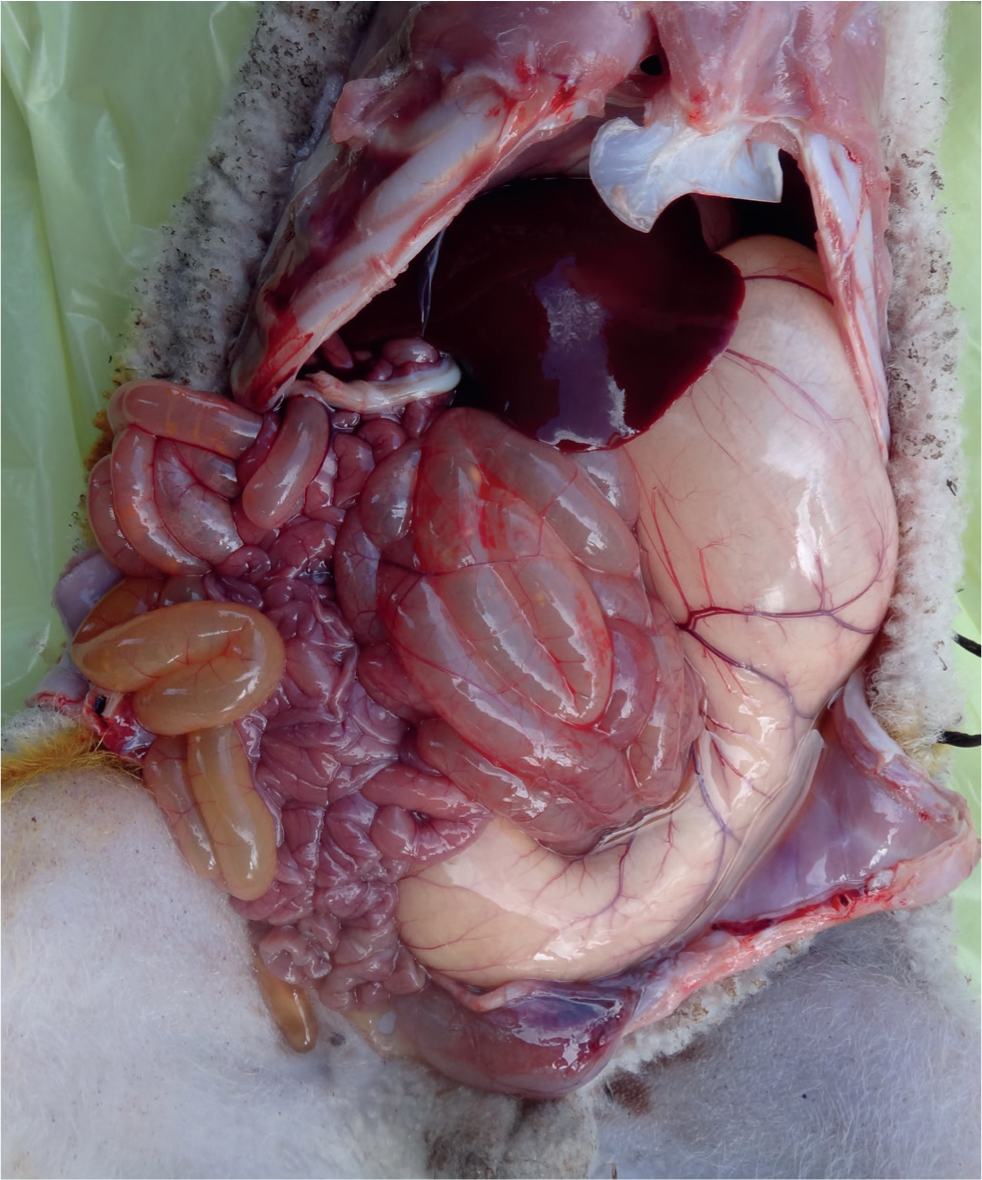
Many cachectic lambs will salivate in extremis (Linklater, 1989) but usually less so in those with WM; therefore, in the terminal stages of disease diagnosis may be more difficult. However, in earlier stages and by observing the progression of individual cases, diagnosis presents few difficulties. Diagnosis may be clarified post mortem where abomasal distension with gas and saliva together with a possible retained meconium may be observed (Figure 3) (Carson, 2019).
Differential diagnoses include colibacillosis (neonatal lamb diarrhoea) and lamb dysentery (Mitchell and Linklater, 1983; Sargison, 2008); salivary abomasum disease (SAD) (Christodoulopoulos et al, 2013); and lamb D-lactic acidosis syndrome (LDLAS), also known as drunken lamb syndrome (DLS) (Angell et al, 2013). These diseases can usually be differentiated clinically from WM by careful observation of the progression of the disease and the clinical signs seen.
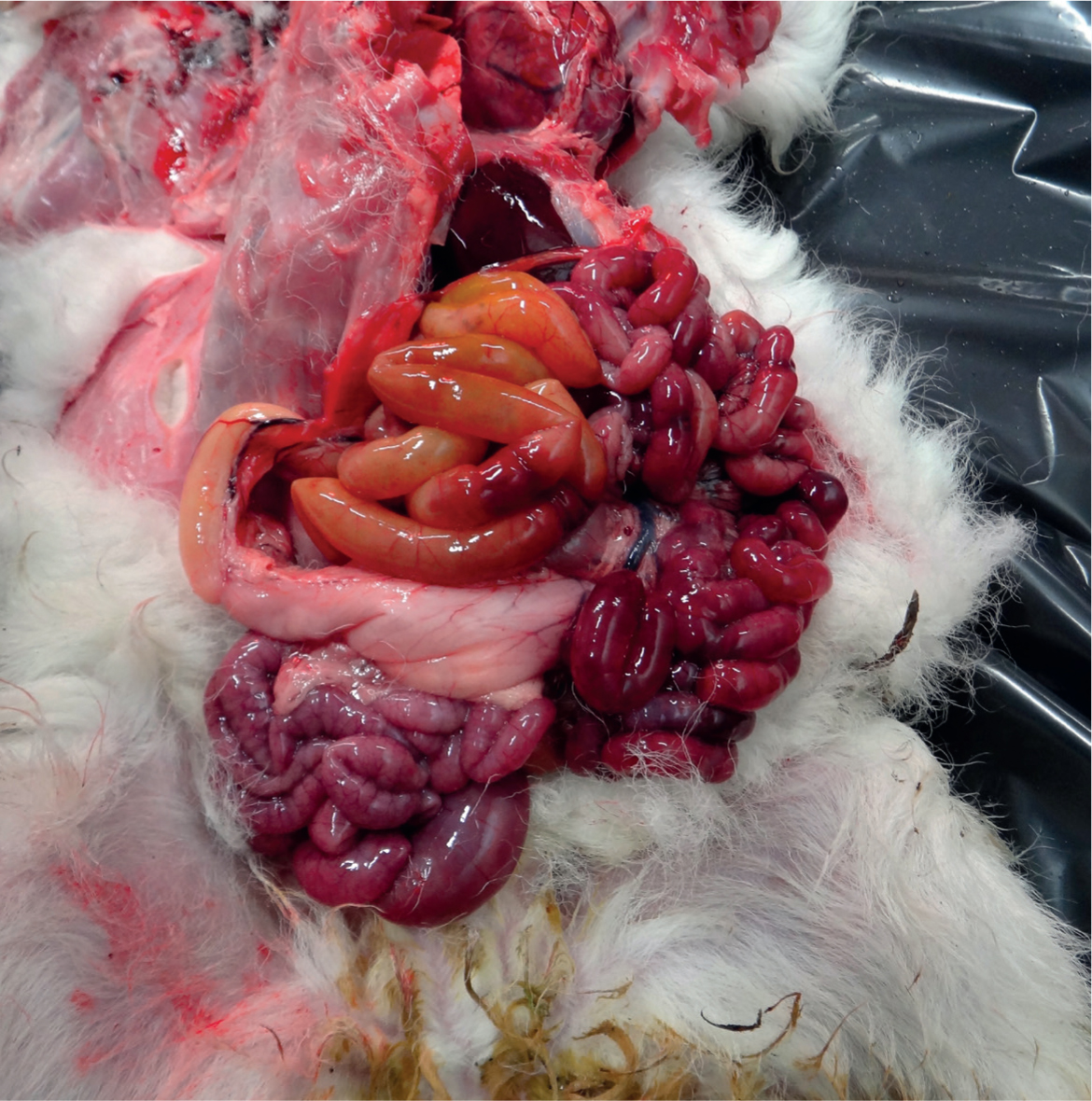
Lamb dysentery manifests as the sudden death of young lambs, usually up to 10 days of age, and more slowly developing cases may produce profuse volumes of blood-stained diarrhoea, although death may occur too rapidly for this to be observed (Sargison, 2008). At post-mortem examination these lambs classically have obvious haemorrhages in the small intestine (Figure 4) and epsilon toxin may be identified.
Lambs affected with SAD tend to be older than those with WM (mean age=7.23 days; SD=3.14), and do not present with clinical or biochemical signs of endotoxic shock (Christodoulopoulos et al, 2013). It has been suggested that SAD is possibly equivalent to WM but presents differently due to the increased age of the lambs (Christodoulopoulos, 2008). To the authors' knowledge there has only been one study of SAD to date and more research is needed to identify the key similarities and differences between SAD and WM.
Some clinical signs are shared between lambs with WM and those with LDLAS; however, those with LDLAS also tend to be older — approximately 2 weeks of age, and do not present with hyper-salivation. Enteritis was noted in two of 10 cases of LDLAS together with nephrotic changes in all 10 cases; however, endotoxaemia was not a feature of the biochemical values (Angell et al, 2013). Furthermore, while acidosis is a feature of LDLAS and possibly SAD, an alkalosis has been observed in some cases of WM (Scott and Gessert, 1996) until the latter stages when a metabolic acidosis results (Hodgson et al, 1989b).
Biochemical and haematological data
The mean biochemical and haematological data reported by Eales et al (1986) revealed no abnormalities, and similar findings were demonstrated by Scott and Gessert (1996). However, in an experimental model, plasma lactate and urea concentrations increased significantly in cases compared with controls towards the terminal stages of disease. Plasma glucose, total protein (mostly the globulin component) and the number of white blood cells decreased significantly in cases compared with controls (Hodgson et al, 1989b; Hodgson et al, 1995).
Pathology
Only one systematic study of pathological changes in lambs with WM was identified (Gilmour et al, 1985) and the results from these 38 lambs are summarised in Table 3. Enteritis was a feature of the disease in 25 out of 38 cases (66%), although the causal nature cannot be ascertained due to the cross-sectional nature of the study. Retained meconium was only present in 12 (32%) and the changes seen in the liver, lung and nervous system appear incidental compared with the changes observed in the alimentary tract.
Table 3. Summary of pathological changes observed in 38 lambs with WM (n=38 unless otherwise specified)
| Body region/organ system and pathological change observed | Number of lambs (percentage of lambs) | |
|---|---|---|
| External examination | ||
| External evidence of diarrhoea | 11 | (29) |
| Rumen | ||
| pH (n=37) | Mean | 5.8 (SE 0.15) |
| Milk present | 1 | (3) |
| Watery content | 37 | (97) |
| Abomasum | ||
| pH (n=38) | Mean 3.9 | (SE 0.15) |
| Milk/clots present | 20 | (53) |
| No milk present | 18 | (47) |
| Inflammatory changes present in mucosa | 1 | (3) |
| Bacteria isolated | ||
| Small intestine | ||
| Empty | 1 | (3) |
| Liquid | 37 | (97) |
| Inflammatory changes present in mucosa (n=25) | 12 | (48) |
| Histological evidence of colostrum absorption | 29 | (76) |
| Large intestine | ||
| Empty | 1 | (3) |
| Liquid | 16 | (42) |
| Firm | 9 | (24) |
| Meconium present | 12 | (32) |
| Inflammatory changes present in mucosa (n=25) | 13 | (52) |
| Inflammatory changes present in lambs with retained meconium (n=12) | 8 | (67) |
| Liver | ||
| Hepatitis associated with ascending umbilical infection | 2 | (5) |
| Fatty degeneration | 4 | (11) |
| Lung | ||
| Suppurative broncho-pneumonia | 1 | (3) |
| Mild interstitial pneumonia | 9 | (24) |
| Retention of some fetal characteristics | 4 | (11) |
| Nervous system | ||
| Mild non-suppurative meningoencephalitis | 1 | (3) |
| Perivascular lymphocyte cuffs in the coeliacomesenteric ganglion | 2 | (5) |
In this study there was no correlation between WM and low colostrum absorption, although immunoglobulins were not assessed by methods other than histology. In the study by Collins et al (1985), lower values of immunoglobulin G may have been associated with an increase in mortality, but there was wide variation within the small sample (n=23). In a natural experimental model of infection, nine of 18 lambs developed WM and these were reported to have inflammation of the gastrointestinal tract (also distended with gas), pale kidneys and muscle, a dehydrated carcass and enlarged mesenteric lymph nodes (Hodgson et al, 1999).
In a single case from New Zealand, the abomasum was pale, gas filled and contained a small milk clot and clear mucoid fluid. The small intestine was also pale and gas filled, and contained sparse contents. The bladder serosa was congested and the pericardium contained 10 ml of serous fluid (Sargison et al, 1997). Similarly, Shaw (1981) reported that the abomasum usually contains an excessive quantity of clear mucous, which may or may not be mixed with the colostrum/milk and there may be a retained meconium.
In comparison, abomasal haemorrhages, abomasal bloat and the presence of excess saliva in the abomasum were a feature in cases of SAD and some also had nephrosis (Christodoulopoulos, 2008), but neither abomasal haemorrhages nor nephrosis were reported in the WM cases by Gilmour and others (1985). In LDLAS the pathological picture was also different to WM, with nephrosis a feature in all 10 cases and mild enteritis only identified in three of 10 (Angell et al, 2013).
Bacteriology
In an observational study, Gilmour et al (1985) reported a bacteraemia at the point of euthanasia, with E. coli identified in 14 of 20 (70%) lambs, and culture of abomasal and intestinal samples revealed coliform, Clostridiium and Streptococcus species to be most prevalent. In another observational study, positive bacterial cultures from blood samples from 71 cases (38%) demonstrated coliforms (31%), Staphylococcus spp. (30%) and Bacillus spp. (20%) (Eales et al, 1986). A pure growth of E. coli (without the K99 antigen) was also identified from the small intestine, liver and pericardial fluid in a single case from New Zealand (Sargison et al, 1997).
In experimental studies, colostrum-deprived lambs reared in a contaminated environment were shown to frequently develop a bacteraemia within 2 to 12 hours of birth and a bacteraemia was a persistent feature of the lambs with WM compared with controls, with all the isolates identified as coliforms (Hodgson et al, 1992; 1999). A bacteraemia was also demonstrated in gnotobiotic lambs experimentally inoculated by mouth using an E. coli strain isolated from a clinical case (Hodgson et al, 1995). However, this bacteraemia was more intermittent and of lower concentration (cfu/ml) in lambs given human endotoxin-core hyperimmune IgG before inoculation compared with those given a saline control. In comparison, E. coli were only cultured from six of 37 abomasal samples from cases of SAD (Christodoulopoulos et al, 2013) and were found to be prevalent in cases of LDLAS (Angell et al, 2013).
Aetiology and pathogenesis
The specific aetiology of WM is unknown and, indeed, there may not be a specific aetiological agent. The pathogenesis so far has only partially been unravelled and there remain key gaps in our knowledge, but three key features appear pertinent and are likely to play a role in the pathogenesis: 1) the ingestion of E. coli from a contaminated environment; 2) the production of endotoxin; and 3) a delay in abomasal emptying.
Biochemical analysis has shown in both observational and experimental studies that endotoxins, possibly released as a result of lysis of large numbers of Gram-negative bacteria, play a role in the pathogenesis and are responsible for the majority of the clinical signs (Hodgson et al, 1989a; 1989b; Hodgson, 1993; Sosa et al, 1994; Hodgson et al, 1995). Experimental work has shown that E. coli serotypes not currently considered ‘pathogenic’ may cause the disease when orally inoculated into gnotobiotic lambs. However, there have been no comprehensive bacterial surveys of the E. coli serotypes associated with WM and it is currently unclear whether any E. coli can cause the symptoms seen given the right conditions, or whether certain pathotypes are more commonly associated with disease. The effects of endotoxin are well studied and have been reviewed extensively by Hodgson (2006). It can cause hypoglycaemia, reduce body temperature, depress appetite and induce gut stasis and lead to death via multiple critical organ failure (Hodgson, 1993). Specifically, endotoxin has been shown to have the following effects on neonatal lambs.
Effects on body temperature
In three lambs aged 4 to 11 days, E. coli derived endotoxin was shown (p<0.05) to accelerate the rate of decline in summit metabolism — that is lambs found it harder to keep warm compared to control lambs (Alexander, 1970). In another study, endotoxin derived from E. coli induced a smaller rise in temperature in lambs compared with ewes when administered intravenously (Coceani et al, 1995) and failed to elicit a significant change in temperature compared with saline-treated control lambs (Goelst et al, 1992). These findings may explain why many lambs clinically appear normothermic with WM (Collins et al, 1985; Eales et al, 1986).
Effects on the cardiovascular system
Sosa et al (1994) administered very large doses of E. coli derived endotoxin (0.5 mg/kg) to neonatal lambs (aged 0–3 days) and studied the effects on ventricular contractility. The mean arterial blood pressure decreased as a result of a decline in myocardial contractility. The adverse effects were shown to be stabilised with saline boluses, resulting in survival of these lambs compared with those that did not receive saline. However, this study used much larger doses of endotoxin compared with the concentration observed (240 ng/ml) as a result of experimental infection with orally inoculated E. coli (Hodgson et al, 1989a) so these effects of endotoxin are likely to be much less in a natural infection. (Working on a circulating blood volume of 60 ml/kg (Swenson et al, 1993) and a weight of 4 kg for a newborn lamb, a lamb may have an approximate circulatory volume of 60 x 4=240 ml of blood. A dose of 0.5 mg/kg is 4 x 0.5=2 mg, so 2 mg/240 ml=0.008 mg/ml or 8000 ng/ml. This is approximately 33 times more than was observed in an experimental infection.)
In a similar study, Bech-Jansen et al (1972) did not show any change in arterial blood pressure, but the follow-up in that study was only for 30 minutes and, when compared to the study by Sosa et al (1994), this was not long enough to detect an effect. Feng et al (2010) showed that cerebral blood flow could be reduced following the administration of E. coli derived endotoxin, but they used much higher doses (10 times) than may be detected in cases of WM. However, even a minor dysregulation of the cerebral blood flow could exacerbate clinical signs, accelerate decline and reduce the chances of recovery.
There is good evidence for the role of endotoxins in WM. The administration of human IgG polyclonal antibody to endotoxin core before the intravenous infusion of E. coli was successful in preventing clinical, biochemical and haematological signs of WM in five lambs, compared with three control lambs infused with human plasma albumin (Hodgson et al, 1995). In that study, none of the five lambs pretreated with the polyclonal antibody developed signs of WM, but two out of three control lambs did.
Delayed abomasal emptying has been observed clinically (Collins et al, 1985) and this was confirmed in an experimental study comparing 34 cases of WM with 68 healthy controls (Eales et al, 1985). In this study, contrast radiography with barium sulphate was used to compare the rate of abomasal emptying. Delayed abomasal emptying was observed in healthy lambs aged 24–48 hours, but gut tone and motility were depressed to a greater extent in the lambs with WM (p<0.05). More recently, the acetaminophen absorption test was used experimentally to investigate the effects of an infusion of endotoxin (2 µg/kg) in lambs aged 1–3 weeks (Mozaffari et al, 2018). In that study, the mean CMAX, TMAX and AUC were lower (p<0.05) for the five treatment lambs compared with the five control lambs, suggesting delayed abomasal emptying as a result of the endotoxin.
An aetiopathogenic hypothesis
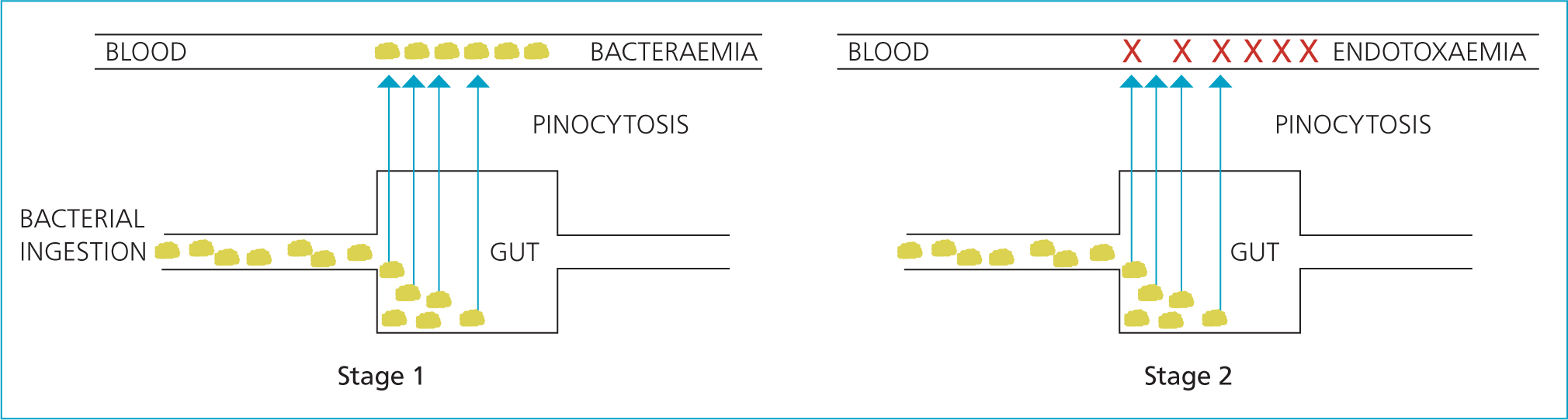
The integration of this evidence has led to the development of the following aetiopathogenic hypothesis. The abomasal contents of the newborn lamb is relatively neutral and the gut has relatively reduced motility, which may serve normally to assist in the uptake of colostrum-derived immunoglobulins (Eales et al, 1985; Leek, 1993). Paradoxically, this may also allow the survival and growth of bacteria ingested from the environment, especially in the absence of colostrum, and this may be compounded by the effect of bacterial endotoxin. The normal pinocytotic mechanisms, which exist for the first 24 hours of life to transport large molecules from the gut into the systemic circulation, may allow access by bacteria and result in a bacteraemia (Hodgson et al, 1989a; 1992). Some bacteria may express factors that opportunistically assist in this process, such as the aerobactin-mediated iron transport system (Der Vartanian et al, 1992). Lysis en masse of Gram-negative bacteria may lead to large volumes of endotoxin, resulting in an endotoxaemia and its clinical sequelae (Figure 5) (Hodgson et al, 1989b; King and Hodgson, 1991; Hodgson, 1993; Sosa et al, 1994; Mozaffari et al, 2018).
Epidemiology
Frequency and distribution
WM has been reported widely in the UK, but there have also been reports from France (including a similar condition in neonatal goat kids) (Savey, 1986; Poncelet, 2004), Spain (Garcia de Jalon et al, 1990), Canada (Hodgson, 1993), New Zealand (Orr, 1995; Sargison et al, 1997) and Norway (Phythian, personal communication). It seems likely that it could occur anywhere where sheep are produced under intensive conditions.
Accurate, up-to-date data on prevalence or incidence rates at regional or national level are unavailable, although personal experience and numerous discussions with practitioners would suggest that WM is widespread, with large variations between farms and within farms from year to year. Unpublished pilot work with one large UK veterinary practice in 2015 revealed that 65% of 895 farmers used some form of prophylactic or metaphylactic antibiotic medication to prevent or mitigate the losses caused by WM, implying that this proportion either currently see or have seen cases historically.
Davies et al (2017) reported 47% (95% CI=41–62%) of 207 flocks (convenience sample) located in England and Wales used licensed oral antibiotics for treatment/prophylaxis of colibacillosis, with a further two flocks prescribed oral antibiotic tablets.
Historically, Collins et al (1985) reported an incidence rate on six farms of up to 3.1% and the mortality of affected cases as 41%, although mortality was observed to worsen with age of onset with those aged more than 48 hours when infected being less likely to recover. Eales et al (1986) reported an incidence rate of up to 24% of lambs born and mortality of affected cases up to 83%, although in this study there was large variation between the 11 farms with those farms with greater contact with the investigators (Woodhouselee, Glencorse and Longyester) reporting a higher incidence rate and lower mortality of affected cases compared with those with less contact (farms A-H). The initial data reported by Scott (1988) suggest that, in the control groups across eight farms, the incidence rate was reported to be up to 43% of lambs born at which point prophylactic therapy was instigated, and mortality of affected cases was up to 25%, despite treatment. Finally, Hodgson (1993) reported that, from a survey of 170 farmers owning 100 000 ewes, WM was responsible for 23% of deaths among housed lambs.
Factors associated with watery mouth
Risk factors for WM include increased litter size, with triplets at greater risk than twins (Collins et al, 1985) and lambs from multiple litters more likely than from single litters (p<0.01) (Eales et al, 1986). Also, Eales et al (1986) reported smaller lambs to be slightly more at risk (p<0.05) although this difference was not observed in cases when compared with their healthy twin. Lambs born to ewes of poor condition were more at risk, although there may have been some confounding by or interaction with age, which was also associated. Lambs were more at risk when born to ewes aged 1 or 5 years but at lower risk when born to ewes aged three years. Early castration (within the first 60 minutes of life) has also been shown to increase the risk of developing WM, possibly as a result of delayed ingestion of colostrum (Collins et al, 1985). Furthermore, incidence rates can vary considerably from year to year, which might suggest that combinations of environmental and management factors are important (Collins, 1981; Eales et al, 1986).
It has been suggested that these factors combined lead to reduced colostrum intake, increasing the risk of acquiring WM (Eales, 1987; King and Hodgson, 1991). In experimental studies, adequate colostrum intake reduces the risk of WM (Hodgson et al, 1992; 1999), although both Collins et al (1985) and Eales et al (1986) showed no association with colostrum-derived IgG in lamb serum.
WM has been reported to be more common in lambs born to housed ewes, although it can occur in ewes lambed outside (Collins, 1981; King and Hodgson, 1991; Sargison, 2008), and it is hypothesised that wet and insanitary conditions lead to an increased risk of ingesting the pathogen by the lamb. This is corroborated by data from Scott (1988), who reported increased incidence over the lambing period in eight flocks, and is also supported by data from Binns et al (2002) who showed that, after adjusting for other confounders, farmers who failed to bed down the mothering pens used at lambing daily reported higher mortality rates compared withthose who did. Clearly, the epidemiology of WM is poorly understood in terms of the frequency, distribution and determinants of disease; indeed, we are limited mainly to one admirable yet flawed investigation from 1986. The scant available evidence and clinical experience would suggest the disease is widespread and endemic in a majority of flocks. A sound understanding of the epidemiology of WM is required to enable its prevention and control and facilitate appropriate targeted treatment as opposed to the current situation of unsustainable mass antibiosis.
Treatment
There have been no well designed intervention studies and the few reports available have been aimed at targeting the perceived aetiopathogenesis. There are two case reports investigating the response to empirical treatment.
In Eales et al (1986), cases of WM were treated with antibiotics administered enterally (neomycin and streptomycin) and parenterally (amoxicillin), glucose/electrolyte solution administered by stomach tube, together with not feeding milk but leaving the lamb to suck the ewe if and when it recovered. This was an uncontrolled study but was reported to be successful in 89% of cases.
A further report investigated the treatment response following amoxicillin plus clavulanic acid injection, together with intravenous flunixin meglumine and an oral rehydration solution. This protocol was successful in 21 of 23 cases (91.3%) (Scott and Gessert, 1996). Other anecdotes include the use of oral flunixin meglumine in isolation (Mitchell, 1992), paracetamol (acetaminophen) (Nixon, 1991), warm water enemas (Robinson, 1981; Shaw, 1981), laxatives (Watt, 1980; Shaw, 1981) and metoclopramide in attempt to restore or increase gut motility (Mitchell and Linklater, 1983; Scott, 1988).
It is possible that antibiotic treatment of already bacteraemic lambs may result in a more severe endotoxaemia due to the increased release of endotoxin as a result of bacterial death. In two studies (Shenep and Mogan, 1984; Shenep et al, 1985), a rabbit model was used to show that plasma endotoxin concentration could increase to much greater levels following the administration of an antibiotic compared with a placebo — but this could vary depending on the antibiotic used. Clearly, well designed intervention studies are necessary to develop an evidence base for treated cases with WM, including supportive therapy aimed at treating the endotoxaemia and a particular focus on the selection of particular antibiotics and timing and route of administration.
Prevention and control
Hodgson et al (1999) demonstrated that lambs fed either colostrum or ewe milk replacer with the addition of oral spectinomycin as the first feed were prevented from developing WM in a natural infection model compared with lambs just fed ewe milk replacer. In that study, nine of 18 lambs developed WM when fed just milk replacer, but no lambs from the other two groups developed disease. The use of this antibiotic was justified on the basis of its poor absorption from the gut and its bacteriostatic activity, in an attempt to reduce the liberation of endotoxin en masse from lysed bacteria (Shenep and Mogan, 1984; Shenep et al, 1985).
On a single farm, Eales et al (1986) examined two preventive protocols. In the first, they administered a combination of neomycin and streptomycin orally (n=58) and in the second amoxicillin orally (n=46), with both interventions given within 15 minutes of birth. Both interventions were pseudo-randomly allocated and compared with lambs receiving no treatment (n=60 and n=63, respectively). Both interventions were shown to prevent WM compared to the control lambs (p<0.001 and p<0.05, respectively), although the study is weakened by the lack of blinding, the possibility of bias during randomisation and the lack of control substance/placebo in the control lambs. Further analysis using the T test suggested that, while the four farms that used antibiotics prophylactically had lower incidence rates compared with those that did not (mean=1.1% SD=0.47), of lambs born (p=0.03), they had relatively high mortality rates in affected cases (mean=59.5% SD=30.0) of cases (p<0.001).
Metoclopramide has been used prophylactically as a preventive in a placebo controlled study of eight farms. On four of these farms there was a reduced incidence compared with the placebo, but in one farm the association was reversed. In the other three flocks the incidence in both groups was too low for comparisons to be made (Scott, 1988). On the basis of these small studies, veterinary advice has included recommending adequate colostrum intake, reducing exposure to infection by ensuring clean dry bedding, and cleaning and disinfecting lambing pens between occupants, and administering oral antibiotics prophylactively/metaphylactically within 15 minutes of birth. Much of this advice remains sound, although the routine prescribing of antibiotics for prophylactic/metaphylactic use is now clearly unsuitable and irresponsible (Sheep Veterinary Society, 2017).
Vaccination
A vaccine would be an attractive option, but the onset of disease is so rapid in clinical cases that a vaccine administered to lambs at birth is unlikely to be efficacious. Vaccinating the ewe would be possible to boost immunoglobulins in colostrum if specific E. coli were associated with disease, as has been shown for some pathogenic E. coli (Cameron and Fuls, 1970; Sojka et al, 1978; Acres et al, 1979; Morris et al, 1980; Altmann and Mukkur, 1983; Gregory et al, 1983; Wray et al, 1983; Pugh and Wells, 1985), but this could be unsuccessful if lambs still fail to get colostrum.
Alternative strategies for prevention
Sodium chlorate was shown to reduce the number of generic E. coli in the colon of treated lambs compared with controls, although the effect seen was weak (p=0.06), possibly due to the low power of the study. However, this option is unlikely to be pursued due to the risks of toxicity (Taylor et al, 2012).
Two other intervention strategies worthy of investigation include the use of probiotic preparations (Bilková et al, 2013; Zhakupova et al, 2017) and the use of bacteriophages (Smith and Huggins, 1983; Johnson et al, 2008). Currently, the normal development of the ovine gastrointestinal microbiome is unknown, but fostering the development of a healthy microbiome may serve to outcompete pathogenic species or the overgrowth of less desirable commensal species. This has been attempted with some success in experimentally reducing the growth of E. coli with the K99 antigen and may warrant further investigation (Bomba et al, 1997; Timmerman et al, 2004). Studies of the microbiome would also serve to inform whether the use of bacteriophages may be worthy of exploration. Several studies again looking specifically at known pathogenic E. coli have shown promising results in neonatal ruminants including lambs (Smith and Huggins, 1983; Johnson et al, 2008). To be successful, specific E. coli associated with WM would need to be identified in order to investigate possible phage candidates as targeted therapeutics.
Conclusions
The clinical picture of WM appears well described and the pathophysiology has been elegantly demonstrated to be attributed to the production of endotoxin, probably as a result of undefined, previously considered apathogenic E. coli. The reasons for these agents becoming established and causing disease in lambs are unknown, although early ingestion from an insanitary environment is plausible. However, the pathology and epidemiology are poorly understood, in turn hampering prevention and therapeutic investigation. We believe WM to be widespread, endemic and largely ignored due to the routine use of prophylactic and metaphylactic antibiosis, a state of affairs that needs to be addressed immediately.
Given the need to improve animal welfare, improve food security, develop farm profitability and reduce antibiotic use in food producing animals, there is an urgent need to assess the current incidence of WM, develop a rigorous understanding of the farm, animal and environmental determinants of disease and develop appropriate and effective non-antibiotic approaches to disease prevention and control. In addition, further investigation using newer technologies of the developing microbial communities within the neonatal gastrointestinal tract may help determine if there are specific pathogens associated or if disease actually occurs due to a deviation from normal physiology or the developmental microbiology. From an animal welfare perspective, well designed treatment studies are warranted in order to treat sick cases appropriately and effectively.
KEY POINTS
- Watery mouth disease in lambs is associated with an endotoxaemia produced as a result of lysis of Gram-negative bacteria.
- Lambs tend to be affected within the first 72 hours of birth and the disease is fatal if left untreated.
- The evidence is weak, however it is likely that disease can be prevented through optimising passive transfer of immunoglobulins at birth through optimal colostrum delivery, as well as ensuring a hygienic lambing environment.
- Prophylactic antibiosis in order to prevent disease is not recommended, although treatment of affected individuals may have some benefits alongside supportive therapy aimed at treating the underlying endotoxaemia.
- Further work is needed to understand if there are any particular aetiological agents involved and to develop prevention, control and treatment strategies.


