It is difficult to define the true cost of liver fluke infection to UK farming, but the Agriculture and Horticulture Development Board (AHDB) estimated a cost of £6 per infected lamb and £96 per infected beef calf (AHDB, 2012). At the herd level, Fasciola hepatica infection results in an estimated 12% and 6% reduction in net profit on an average dairy and beef unit, respectively (Shrestha et al, 2020). Changing weather patterns in the UK over recent years have often resulted in milder winters and summers that may be hot or wet but are above all unpredictable, affecting the epidemiology of liver fluke (Howell et al, 2023).
Lifecycle and epidemiology
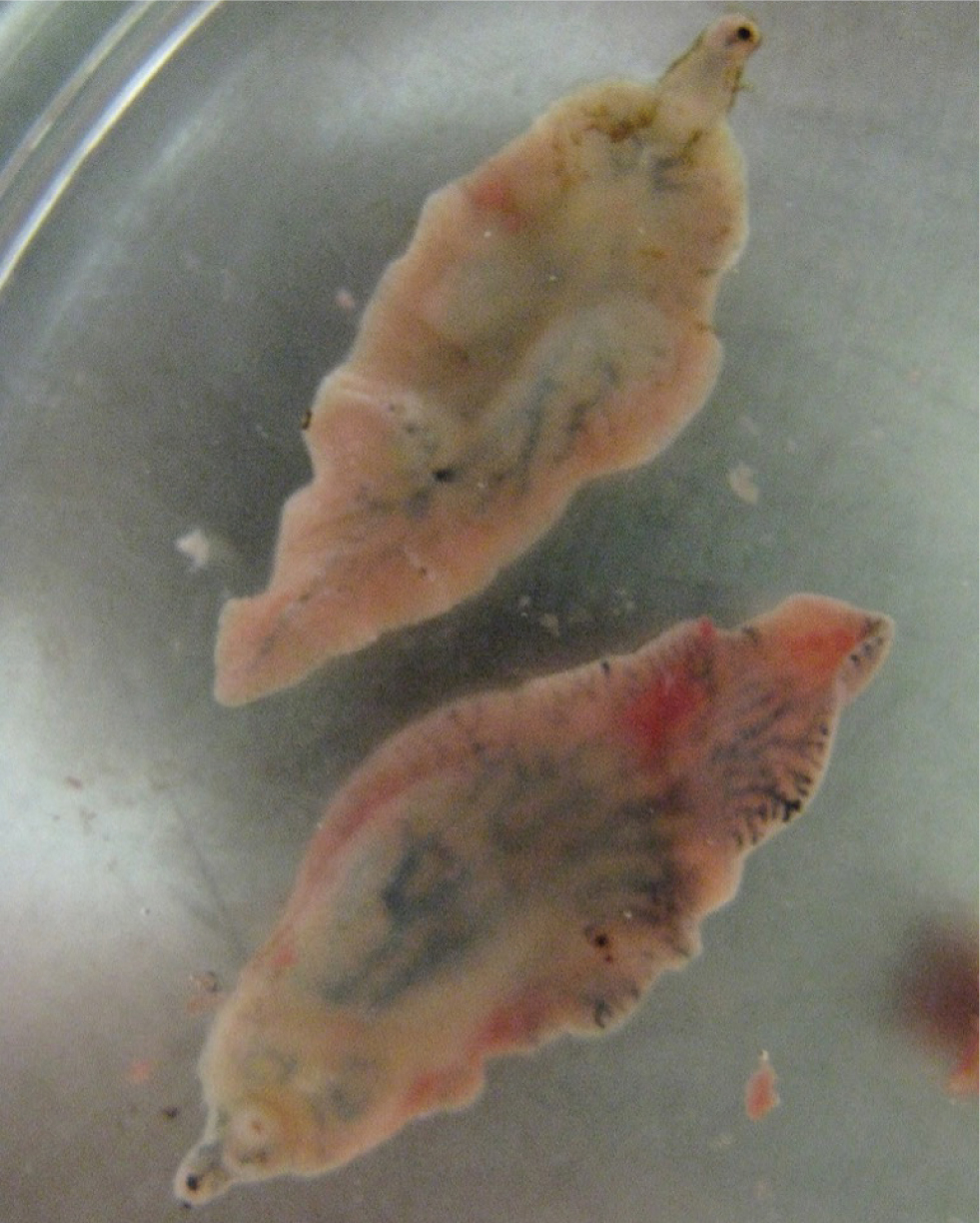
The liver fluke life cycle takes around 20 weeks to complete and requires a snail intermediate host (Figure 1). Mature flukes (Figure 2) reside in the liver of the definitive host. Fluke eggs are passed out in the faeces of infected livestock and develop on pasture. This development is temperature-dependent and takes 2–4 weeks. Eggs then hatch to release miracidia that seek out a host snail. After burrowing into the snail via its foot, the parasites undergo clonal division in the snail body cavity over several weeks, producing several thousand genetically identical cercariae that are then released from the snail (Hodgkinson et al, 2018). The cercariae encyst on plant matter as metacercariae, which are the infective stage. When eaten by a grazing animal, the metacercariae excyst and the young flukes migrate through the gut wall into the liver. It then takes a further 10 weeks for fluke to mature and produce eggs that can be detected in faeces.
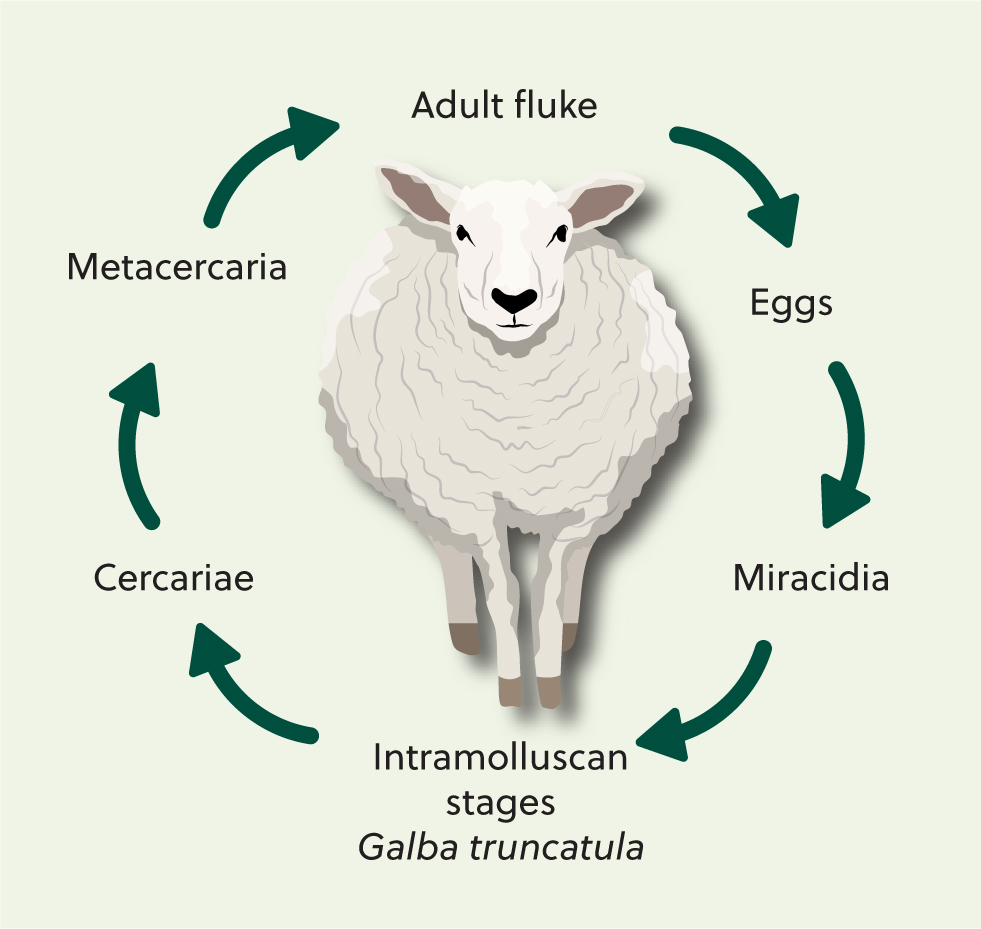
In the UK, the main intermediate host of liver fluke is Galba truncatula, a mud snail measuring 2–5 mm in length (Figure 3). Others snail species, such as Radix and Succinea, may be involved in transmission in acidic moorland soils (Relf et al, 2009). Common habitats include poorly drained areas as well as the banks of ditches, ponds and streams (Figure 4). Poaching because of animal movements can create exposed mud around these features which allows algae, G. truncatula's main food source, to grow.


Fasciolosis is a seasonal disease in the UK, with mean daily temperatures above 10°C required for the development of the free-living and intramolluscan stages. This has traditionally been the case between May and October, although climate change is extending the window of parasite transmission. If weather conditions are wet over the summer months, large numbers of metacercariae are then released from snails onto the pasture between August and October; conversely, extreme hot and dry conditions in spring or summer, as seen in some recent years, can lead to a snail population crash and a subsequent low risk of liver fluke – unless a further period of mild wet weather allows snails to recover before cooler weather sets in again.
F. hepatica can infect a range of animals as well as sheep and cattle. In the UK, rabbits, hares, deer and horses have been found to be infected. Genetic studies have shown that F. hepatica isolated from sheep, cattle and horses come from the same population (Beesley et al, 2017; Howell et al, 2020). This has implications both for parasite control and for the spread of drug-resistant fluke.
Effect on the host
Sheep are more susceptible to the effects of liver fluke infection than cattle. Acute fasciolosis, often seen in the autumn, is the result of blood loss and liver damage caused by high numbers of young migrating fluke following consumption of heavily contaminated herbage. If smaller numbers of infective metacercariae are ingested over a longer period, chronic fasciolosis is the likely outcome and is usually seen in winter or spring. Typical clinical signs are weight loss and anaemia. Cattle are more resilient as, in addition to possessing a larger liver and hence a greater reserve of functional liver tissue, the strong fibrotic reaction elicited by the migrating juvenile flukes prevents the long-term survival of a large proportion of their number. Hence, while acute deaths can occur following a particularly severe challenge, the more common clinical picture is of chronic fasciolosis or subclinical disease (Behm and Sangster, 1999). Weight gain, fertility and milk production can be impaired, even with low fluke burdens. Individual flukes can live for months to years in the host if left untreated, and sheep and cattle do not develop protective immunity against liver fluke. Chronic infection can have an immunosuppressive effect on the host and increase susceptibility to other conditions, such as Salmonella dublin (Aitkin et al, 1979; Moreau and Chauvin, 2010).
Diagnosis
As a result of the increasingly unpredictable nature of fasciolosis outbreaks, as well as resistance to flukicidal drugs, livestock producers are being encouraged to test before treating (COWS, 2023). Treating at traditional risk periods could result in treatments being given too early, wasting money and leaving animals vulnerable later on. There are several tests available; the best test will depend on the time of year and the type and age of animals at risk.
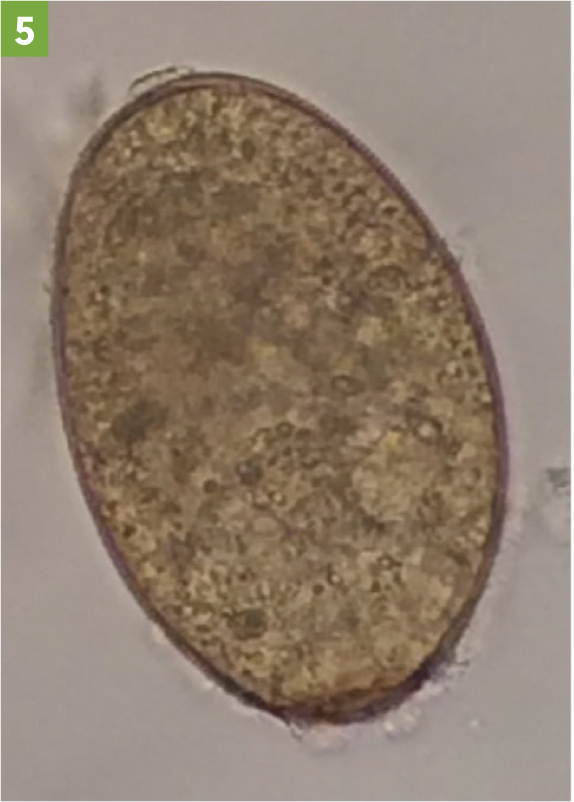
Detection of fluke eggs in faeces by faecal egg counts (FEC; Figure 5) demonstrates the presence of live adult fluke within the host and so are most useful in winter, spring and early summer. They can be used on individual faecal samples or composite samples (Daniel et al, 2012; Graham-Brown et al, 2019). FEC are useful to confirm that treatment has been effective, if a second sample is tested 3 weeks after treatment. However, they cannot detect early infections (before about 10 weeks) and hence cannot be used for the diagnosis of acute fasciolosis. They can also have low sensitivity if used on single animals or on small volumes of faeces, particularly in cattle. Testing ten individuals and taking at least 10 g of faeces per animal helps ensure adequate sensitivity (Graham-Brown et al, 2019).
The coproantigen enzyme-linked immunosorbent assay (ELI-SA) detects enzymes secreted by fluke in faeces (Mezo et al, 2004). It can give a positive test around two weeks before FEC, and can also be used to confirm efficacy of treatment (Flanagan et al, 2011; Gordon et al, 2012). Individual faecal samples should be taken and tested separately. Ten individuals from a larger group can be tested to inform treatment of the group (COWS and SCOPS, 2022; Colston and Mearns, 2023). The coproantigen ELISA is most useful from winter until the following summer.
Serum antibody detection ELISAs are most useful for monitoring exposure in lambs in their first grazing season and should be used on farms that routinely give autumn fluke treatment. Samples should be collected from ten lambs per management group each month from late summer until seroconversion (COWS and SCOPS, 2022; Colston and Mearns, 2023). Lambs seroconvert 2–4 weeks following infection, meaning a positive antibody test in first season grazing lambs is useful to target treatment at the right time. The same principle applies to calves if they are at risk of acute fasciolosis. Serum antibodies persist following treatment, so testing serum cannot be used to assess treatment efficacy, and false positives may sometimes result when testing older animals with prior exposure. The sensitivity and specificity of antibody-detection ELISAs is high, at over 80%.
Bulk milk tank testing detects antibodies and can be done a few times a year to monitor infection levels within a herd. Individual milk antibody testing is a useful way to identify whether a dairy cow is infected, for example if considering treatment at drying off. The limitations are similar to serum antibody testing.
Post-mortem examination of suspect fallen stock as well as abattoir reports of liver fluke found in the livers of slaughtered animals can also provide a useful, free source of information.
Treatment and control
Triclabendazole, resistance and other flukicidal drugs
Triclabendazole is unique in its efficacy against immature fluke that are from 2 days old in sheep and 2 weeks old in cattle. Historically, a lack of diagnostic tests available for early fluke infection meant that sheep on high-risk farms were repeatedly dosed with triclabendazole in the autumn to avoid the risk of acute fasciolosis, which has unfortunately led to resistance. A recent prevalence study showed that triclabendazole resistance was present in north-west, north-east and south-west England and in Wales (Kamaludeen et al, 2019).
Triclabendazole requires partial metabolism by the liver in order to work, so it may be ineffective in severely parasitised individuals, or those with concurrent liver disease from another cause. Apparent flukicide failure can also occur as a result of underestimation of bodyweight or uncalibrated equipment. If in doubt, doing a faecal egg count reduction test (FECRT) to confirm the level of resistance to triclabendazole is advisable (Box 1; Daniel et al, 2012).
How to perform a faecal egg count reduction test (FECRT) for liver fluke resistance to triclabendazole
Eggs must be present in faeces in order to do the test.
Day 1:
Day 21:
Repeat the faecal sampling in the same way, from the same individual sheep as before.
A drop in egg numbers of 90–95% or more indicates good levels of efficacy, while any less suggests that resistance is starting to develop.
The copro-antigen reduction test works on similar principles: see https://www.scops.org.uk/internal-parasites/liver-fluke/tackling-resistance/ for further details.
Livestock producers are now advised to minimise their reliance on drugs and, when a flukicide is required, to choose one that is appropriate for the age of parasite likely to be present at the time, avoiding over-use of any single active ingredient. In practice, this means triclabendazole is the drug of choice in the autumn for animals at risk from acute fasciolosis, with closantel another option at this time of year. For housed cattle or for treating sheep in the spring, one of the other products active against adult stage parasites is preferable. Table 1 shows the licensed flukicide products available in the UK. The plethora of different brands and formulations of these few active ingredients can sometimes cause confusion over which active is being used.
| Active ingredient | Minimum age of fluke killed (sheep) | Minimum age of fluke killed (cattle) | Optimum time of year to use | Use in dairy cattle (guide only – check product data sheets) |
|---|---|---|---|---|
| Albendazole | 12 weeks | 12 weeks | Spring | 60 hours milk withhold |
| Oxyclozanide | 12 weeks | 12 weeks | Spring | 108 hours milk withhold |
| Clorsulon | N/A | 12 weeks | Spring | Use at beginning of dry period only |
| Rafoxanide | 12 weeks | 12 weeks | Spring | Not licensed |
| Closantel | 5 weeks | 7 weeks | Autumn | Not licensed |
| Triclabendazole | 2 days | 2 weeks/6–8 weeks (depending on formulation) | Autumn | Oral only. Use at beginning of dry period only |
Currently there is no resistance reported to the other flukicides in the UK, but this is always a risk, particularly for closantel as the second-best option for treating immature stages. In addition, frequent use of closantel may increase the risk of resistant Haemonchus species (barber's pole worms) emerging, if these are present on farm. There are no new drugs or vaccines entering the market in the near future, making it vital to use the available drugs responsibly. The concept of preserving a population of parasites in refugia (leaving some of the flock untreated) is advised for control of gastrointestinal nematodes in sheep but is considered too risky for liver fluke because of its high pathogenicity (Hodgkinson et al, 2019).

Rafoxanide is a relatively new re-addition to the market in the UK, although it has been used elsewhere in the intervening period. There is evidence of cross-resistance between closantel and rafoxanide (Swan, 1999), therefore rafoxanide should not be used as an alternative to closantel.
Control programmes
Control programmes should be farm-specific and take into account whether triclabendazole resistance is present. Controlling fluke is complicated, so veterinary involvement is essential. Because of the seasonal nature of fasciolosis it is helpful to divide the year into seasons for planning purposes.
In spring, the focus should be on the prevention of pasture contamination. Sheep and cattle should be tested to see if they are infected, if they were not treated during the winter. A composite FEC is a convenient way of doing this. If treatment is necessary, something that is effective only against adult parasites should be used. Spring is also a good time to perform a FECRT to ascertain triclabendazole resistance if egg counts are high enough.
Autumn is the high-risk period for acute fasciolosis; as far as possible, avoid grazing wet pasture at this time of year. If sheep are on the farm, or if cattle are at high risk, use the serum antibody test monthly from late summer to determine when and whether to treat. None of the flukicides have any persistent activity, so stock will become re-infected if kept on contaminated pasture, potentially needing repeat treatment.
During the winter months, for housed cattle, testing is advised rather than routine treatment. If FEC are used, it is advisable to wait several weeks after housing and then, if necessary, to use a product active against mature flukes.
The same seasonal principles apply in dairy herds, although treatment can usually only be given during the dry period. Cows should be tested before treatment; the milk antibody test can be used at any time of year. Choice of product, if needed, should be made considering the time of year and the likely ages of fluke present (Table 1). Care should be taken as some products, including rafoxanide and injectable formulations of triclabendazole, cannot be used in dairy replacements of any age.
Reducing liver fluke exposure through pasture management should be considered. Identification of snail habitats is difficult as these can change within and between seasons depending on weather and vegetation coverage. Wet areas with exposed mud are likely to harbour snails unless the soil is very acidic, such as peat uplands. Options to consider include avoiding wet pastures during high-risk periods (autumn), temporary fencing of wet areas during risk periods, permanent fencing of wet areas, or housing of animals during high-risk periods. Infective fluke cysts cannot survive in good quality silage (John et al, 2019). Complete avoidance of wet pastures or flukey areas is often not possible because of insufficient alternative pasture or drinking facilities.
All animals brought onto the farm should be considered to be potential carriers of triclabendazole-resistant fluke, including rams, bulls and overwintering sheep, and should be quarantined accordingly. Current advice from COWS (2018) is to keep incoming sheep or cattle either indoors (preferably) or on a dry pasture until any liver fluke acquired at the previous farm are mature enough to be killed by a flukicide containing an active ingredient other than triclabendazole. For example, closantel can be used for sheep that have been grazing at their farm of origin after a wait period of 5 weeks. Following a further 6 weeks, a FEC or coproantigen test should be used to check treatment has worked before putting out onto fluke risk pasture.
Conclusions
Triclabendazole resistance is increasing and the epidemiology of fasciolosis has changed over recent years. Both of these factors present challenges to the control of the parasite. Testing before treatment and, if necessary, choosing the most appropriate product are required for sustainable liver fluke control.


