At the recently held Responsible use of Medicines in Agriculture Alliance (RUMA) conference, industry achievements to reduce antibiotic use to date were celebrated and future challenges and opportunities were acknowledged.
The morning programme of the conference, which was held online for the first time and attracted over 150 delegates from the UK and overseas, was devoted to domestic issues and focused on the positive story of responsible use of medicines in UK farming and companion animal sectors, which see high standards of food safety, animal health and welfare, and concluded with a look ahead to the UK animal medicines legislation. The afternoon programme looked at the international context for the responsible use of medicines, concluding with a look at how the efforts of UK farmers to develop high standards of food safety, animal health and welfare, create an expectation as the UK develops new international trade relationships.
Commenting on the event, conference and RUMA Chair, Cat McLaughlin says: ‘The conference has shone a spotlight and prompted important debate on the challenges and opportunities that lie ahead.
‘As well as recognising the incredible achievements of the voluntary response to the AMR challenge by the UK's livestock sectors to date, the event also covered the scope and opportunities for the future UK veterinary medicines legislation, responsible use around the world and the global vision for new animal medicines, the story of the UK's high health and welfare standards in international trade discussions, as well as the expansion of the RUMA blueprint into the companion animal and equine sectors with the recently formed RUMA Companion Animal and Equine Alliance. We also heard about key sector initiatives such as Farm Vet Champions and the Medicine Hub, both of which are key to driving best practice and robust data collection.
‘The level of delegate engagement was outstanding and there were a number of key themes that emerged, covering the ongoing importance of robust data gathering to help drive informed decision making, the need for funding to support innovation, the environment, and recognition that industry and sector collaboration is vital in driving ongoing behaviour change.’
Session overviews and commentary
RUMA targets
The conference began with a look at the RUMA Targets highlighting the voluntary response to the antimicrobial resistance (AMR) challenge by the UK's livestock sectors to date to review their use, refine management practices and where sensible, bring about sustainable reductions in antibiotic use in farm animals.
RUMA was established in 1997 to promote the highest standards of food safety, animal health and animal welfare in the British live-stock industry. It brings together organisations from right across the livestock supply chain, and seeks to promote a co-ordinated and integrated approach to best practice in the use of medicines. RUMA champions responsible use of animal medicines in UK livestock by producing specific ‘guidelines on responsible use’ and has a current focus on AMR which is co-ordinated through a range of collaborative activities that contribute to the UK 5-year AMR strategy, most notably driven through the RUMA Targets Task Force (TTF).
Lord O'Neill set a challenge to UK Agriculture in 2016 to respond to the AMR challenge and contribute to the UK's One Health effort to reduce resistance to antibiotics and protect human health. The creation and roll out of the first set of sector-specific targets in 2017 through the RUMA TTF, helped focus activity across the UK livestock sectors to achieve a 52% reduction in antibiotic use since 2014 and, as the second phase of TTF targets shows, that commitment to further reduce where this is sustainable, continues. As a result, less than 30% of all the antibiotics used in the UK, are now used in the billion plus poultry birds, laying hens, sheep, cows, pigs, fish and game produced to feed the nation, and for export, which is a downward shift of about 12% since 2014 (Figure 1).
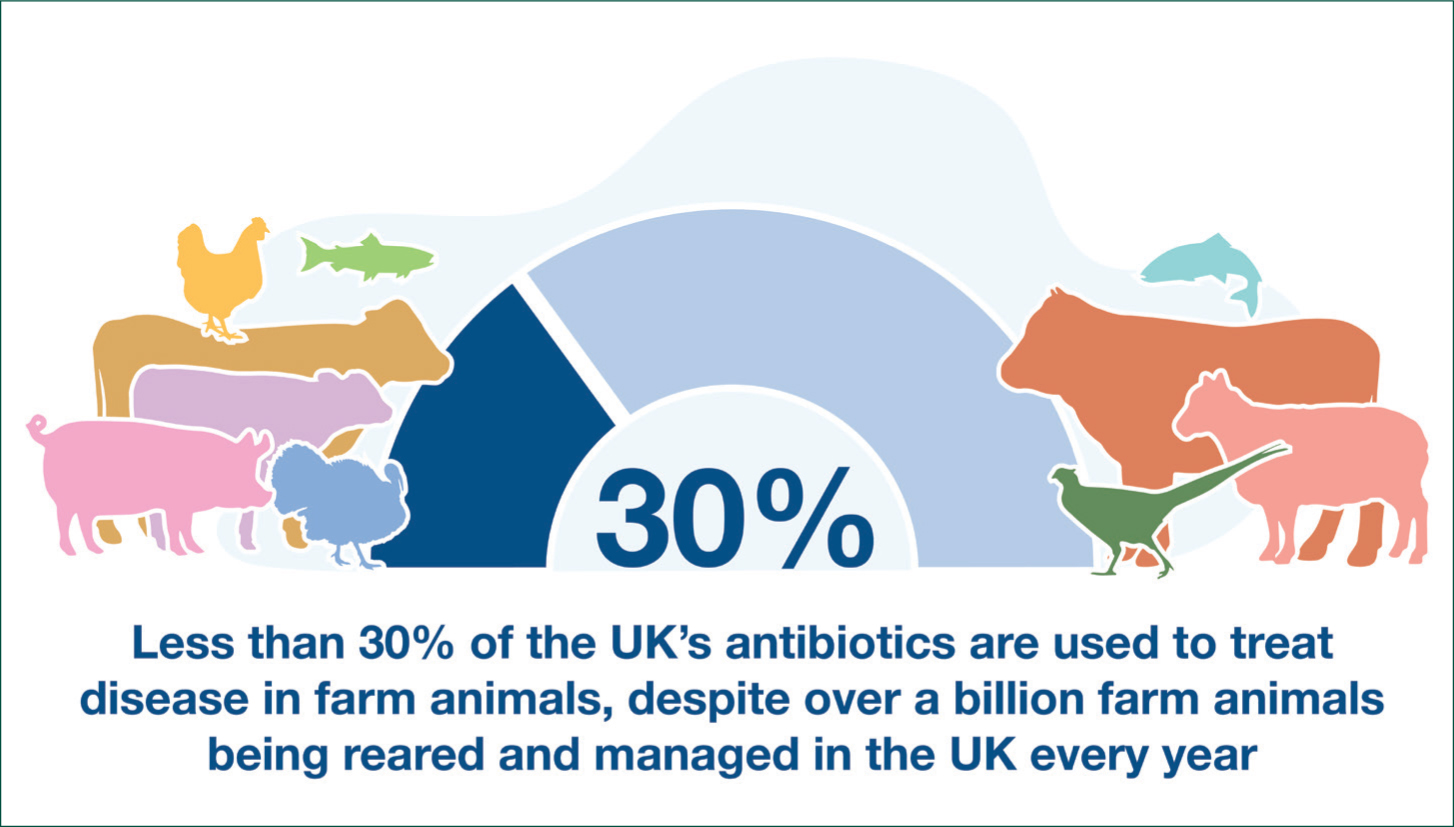
But session chair, Chris Lloyd, RUMA Secretary General pointed out, this is not about working towards zero use of antibiotics. He highlighted the need to ‘have medicines in our cabinet to tackle disease and ensure animal health and welfare and that the key is to ensure responsible use: as little as possible, but as much as necessary.’
These achievements, which were widely applauded by both speakers and delegates at the conference, have been realised principally through voluntary multi-sector collaboration. The first set of targets issued in 2017 became the industry roadmap and focus for everyone along the supply chain and across each of the sectors. In 2021, the second set of targets up to 2024 (developed by the Targets Task Force 2 — TTF2) were released and last year (RUMA, 2021), the 1 year on report was published. It reveals that for some sectors it is still too early in the 4-year targets cycle to provide accurate data, but where data are provided they indicate good first year progress, despite the challenges of the global pandemic and the UK's exit from the EU. The unprecedented nature of the past year has resulted in significant industry challenges including supply chain resource and infrastructure issues, and labour difficulties. This set of unique circumstances have had far-reaching and varying impacts, some of which are yet to be fully understood across the farming industry and the journey towards the targets.
As a result of these global and political impacts some sectors, such as gamebirds, had reduced production and others, such as the pig sector, experienced considerable supply chain issues with animals remaining on farm for significantly longer periods of time than normal. This means that some of the figures reported are not representative of a ‘normal’ year of activity. In addition, the process the sectors have gone through to work towards their targets has in some cases, also been unavoidably affected and caused delays to the development and launch of key initiatives. The full effect this will have on the targets, and how long it will take for these impacts to be felt, understood, and addressed, is not yet known. Equally, the report shows that the onset of environmental issues related to global climate change have had impacts in some sectors too.
Chris Lloyd, RUMA Secretary General said of the latest TTF Report: ‘I encourage you all to digest the report to get an idea of the phenomenal effort undertaken across every live-stock sector to play its part in reducing the risk of AMR in the interest of preserving the future use of antibiotics for animal and human medicine. We hope these targets demonstrate to everyone that the UK livestock sector is still committed to promoting the responsible use of antibiotics and reducing their use where sustainable.’
Chris went on to discuss the importance of data, which was a significant theme and focus during the whole event, saying: ‘What these early experiences have shown is that having data to build the picture of antibiotic use for a sector is really important at two levels: nationally, it helps to show the trend of antibiotic use in the sector; and at a farm level, once data become available, it offers an opportunity for producers to use their medicine use as a discussion tool with their veterinary surgeon to assess areas where refinement of management practices could deliver real productivity benefits on their farm and livestock enterprise. Data help galvanise both industry activity and farm level discussions.’
Spotlight on sector initiatives
The conference also shone a spotlight on a number of important sector initiatives, all of which play a role in supporting the journey towards achieving the latest set of targets. These included the Welsh Beef and Lamb Producers cooperative, which is building data recording into their annual veterinary health and welfare review as part of the Farm Assured Welsh Live-stock assurance rule. Similar steps are being taken across the Red Tractor scheme rules, and the Agriculture and Horticulture Development Board (AHDB) have developed a web-based recording system for the ruminant sectors, called the Medicine Hub, which was launched last year. Avoiding duplication and making it as easy as possible to capture data have been at the forefront of the Medicine Hub development. Another important industry initiative high-lighted at the conference was Farm Vet Champions, which was introduced by Fiona Lovatt, sheep veterinary surgeon and clinical lead for RCVS Knowledge Farm Vet Champions. Farm Vet Champions, a major collaborative project that is spearheaded by RCVS Knowledge and funded by the Veterinary Medicines Directorate (VMD), aims to unite and empower UK farm animal veterinary practitioners to establish good antimicrobial stewardship in practices and on farms.
At the conference, Fiona Lovatt said: ‘Farm Vet Champions is specifically targeted at general practitioner veterinary professionals who are working in UK farm animal practice, but the material is available to any member of the practice team to include veterinary technicians, managers, and reception staff because, although antibiotics are always prescribed by a veterinary surgeon, we are well aware that other members of the practice team are very much involved in interacting with clients and in the actual supply of medicines.’
RUMA Companion Animal and Equine Alliance
Delegates also heard from the recently established RUMA Companion Animal and Equine Alliance (RUMA CA&E). Created from RUMA leadership and vision, this recently formed organisation sets out to establish the principles of responsible use of medicines in the companion animal and equine sectors with a view to contributing positively to the One Health agenda. Inspired by the success of the UK farm animal sector in reducing antibiotic use during the past 5 years, RUMA CA&E will draw on those learnings to help protect important medicines for future human and animal use. This new collaboration will cover the responsible use of medicines in dogs, cats, rabbits, small mammals, exotic animals kept as pets, and equids. The aim is for the UK to lead the way in these sectors through evidence-based and measurable activities that will promote and enhance stewardship. RUMA CA&E will focus initially on the responsible use of antibiotics.
Future scope and opportunities
The future scope and opportunities for UK medicines legislation also featured at the RUMA conference in a discussion between James Russell (Senior Vice President of the British Veterinary Association) and Kitty Healey (Head of Antimicrobial Resistance, Policy & Surveillance, VMD). Kitty talked about the need to look at the regulatory landscape within our country and create legislation that meets our needs and is ‘fit for purpose’. The imminent consultation will consider the programme of legislation, and people were encouraged to participate in the consultation when it begins.
The global picture
The conference also considered the global picture of responsible use featuring insights into the multilateral work going on around the world, in particular through the Global Leaders Group. Discussions centred around surveillance, investor action and voluntary targets, and concluded with a look at the global vision for new animal medicines. The key themes across the speakers centred around disease prevention and the role of vaccines, environment, the critical and growing threat of AMR, and ensuring availability and access to critical medicines when they are needed in the human and animal health world — the right medicines at the right place at the right time.
Christine Middlemiss, the UK's Chief Veterinary Officer, talked about the importance of sharing at a global level, the non-regulatory and successful approach that the UK has taken on AMR because it shows that our food and approaches are safe, and can help lead other countries in their approaches to AMR.
The global discussions at the conference also reflected on the fragility of supply chains particularly in light of COVID-19, both in terms of food but also in terms of medicines and the need for sustainable and affordable supply chains around the world for safe and healthy food and medicines.
Professor Dame Sally Davies, UK Special Envoy on AMR, and Professor J Scott Weese, Ontario Veterinary College, talked widely about the complexities of the AMR challenge and the need for more collaboration between the human and animal sectors, in particular the ongoing need for data, and the fact that while regular linkages are made between the use of medicines in animals and the health of humans, there is limited evidence to suggest their negative impact.
Professor Scott Weese said: ‘From the One Health issue it is the realisation that we live in a complex ecosystem; AMR is an ecological problem, and it is a complex ecological problem, which means it will defy a simple solution. Everyone can do a little bit to contribute a little bit of benefit, but we need everyone to do that for it to work.”
He went on to say: ‘Every time an antibiotic goes into a human or an animal and then comes out of them, we're contributing to this cascade of resistance, so we need to look at the root cause, which is health.’
Dame Sally Davies talked about the importance of data, funding, preventing infections, transforming systems and sustainability, and of getting on top of AMR and mitigating it otherwise ‘…we're going to lose modern medicine.’
She said: ‘Antibiotics underpin the management of infections such as the management of HIV, TB, malaria and other infections, but also transplants, new joints surgery, Caesarean sections. I often say to people, climate change matters, but we'll be dead of superbugs before climate change starts to kill vast numbers.’
Dame Sally Davies summarised by saying: ‘RUMA has led the voluntary work in Britain, and I never thought they could do it; they've done a lot and they're going to do a lot more and it just shows what can be done. Thank you RUMA.’
Conference summary discussions
The conference ended with discussions around the value of the UK's high health and welfare status as the UK seeks to develop and establish new parameters for imports and exports with countries around the world. The importance of data to help the UK continue to evidence its high standards was a key focus, as well as the ongoing importance of scrutiny and transparency in supply chains. Environmental considerations were an ongoing topic throughout the conference, and it was recognised that more data and insight is needed to help shape environmental strategies.
Finally, Professor Peter Borriello, Trustee for Safe Medicines for Animals through regulatory training (SMArt) looked ahead to the future challenges the UK faces in its bid to ensure responsible use of medicines and in response to AMR. In his closing remarks he said he was: ‘…incredibly encouraged by the conference and maybe not for the reasons you would guess, which is the continual reduction in use, the continual reduction away from critical antibiotics and the emergence of the decrease in resistance which is all good news; but the real reason I am encouraged is the very evident emergence of leaders — that is just tremendous.’ He went on to say that: ‘To step up to the plate in your area of responsibility and to be a champion or a leader when you won't get additional time off or remuneration to help drive these changes, takes a lot of effort and for that, everyone should be applauded because without that none of the results would be sustainable’.


