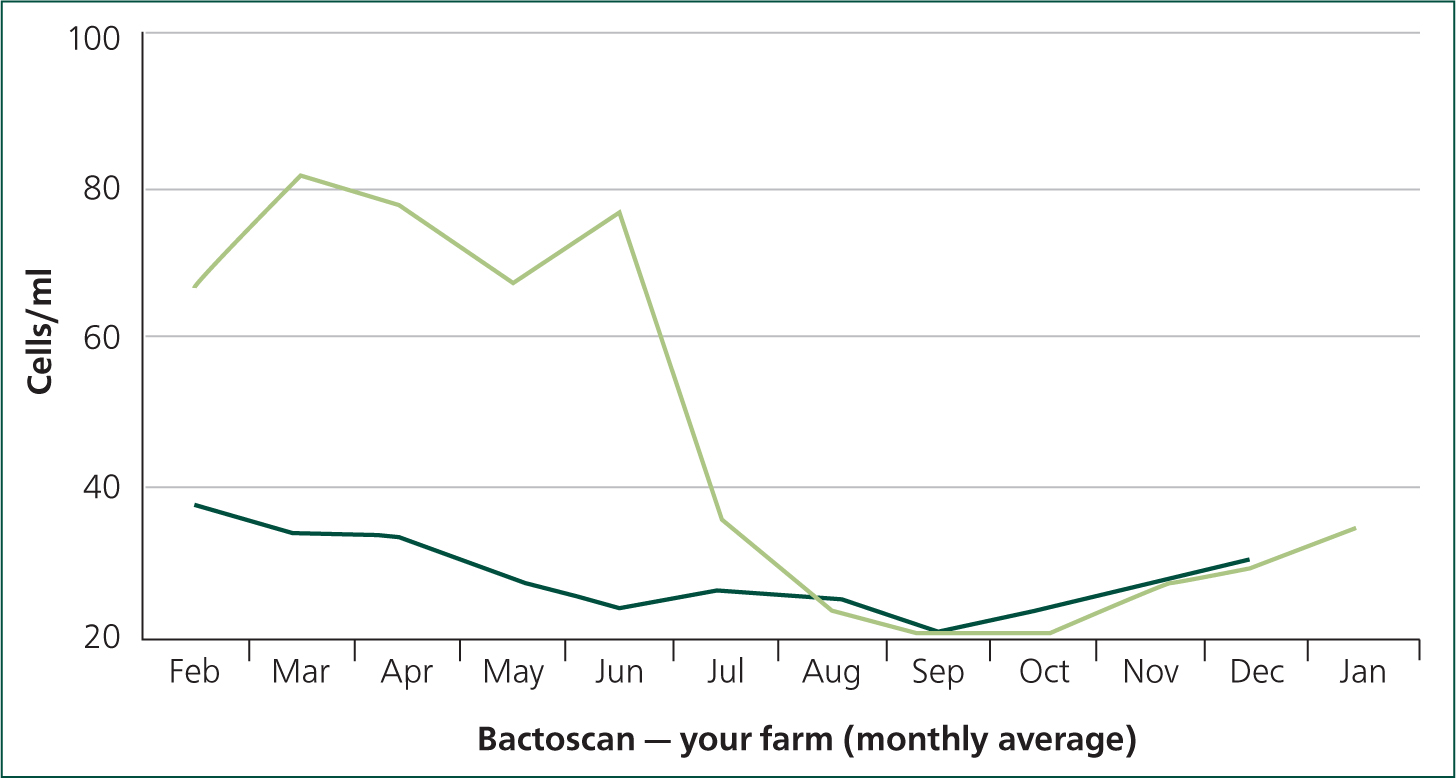
A family-owned, all year-round calving herd of 130 British Friesian cows averaging 6000 litres on a 305-day adjusted lactation seeks advice from the practice regarding their milk quality and Bactoscan, which is ‘always a problem’, but had worsened considerably through the early months of 2020 (Figure 1). The reported clinical mastitis rate is also high at 50 cases per 100 cows/year and herd average somatic cell count (SCC) is increased, with the 12-month rolling average at 218 000 cells/ml.
Lactating and dry cows are pastured in the summer and housed in winter; lactating cows are managed as two groups for winter housing, with a smaller group loose housed on a straw yard, but the majority housed in cubicles bedded with chopped straw (Figure 2). The cubicle housed group have access to a large central ‘loafing’ area, which allows for around 3 m2 of cow ‘living space’ (Thompson et al, 2020). The herd are milked twice daily through a herringbone parlour with a full premilking teat preparation routine. At drying-off, all cows receive an internal teat sealant, with infected cows (>200 000 cells/ml in any of the last three milk recordings) also receiving antibiotic dry cow therapy (Orbenin Extra Dry Cow 600 mg Intramammary Suspension, Zoetis UK Ltd).

The herd milk contract stipulates a rolling 12-month average Bactoscan of less than 30 impulses/ml and the client requests an urgent meeting to discuss milk quality on farm. A senior colleague tasks you with reviewing causes of increased Bactoscan in a dairy herd and you visit the farm to discuss current management and review laboratory results.
Cattle self assesment: QUESTIONS
- Describe the key differences between Bactoscan and total bacterial count (TBC)
- Summarise the likely causes of increased Bactoscan in a dairy herd? Which are more common?
- The client is on every other day collection and harvests milk into two bulk tanks, one ‘larger’ tank that holds three milkings and a ‘smaller’ tank that holds the fourth milking immediately prior to pick-up. The client has therefore submitted a chilled bulk milk sample from both tanks to an independent laboratory for full differential counts and a direct plating (Table 1). Comment on the results.
- Based on discussion with the client from the answers to question 2 and the laboratory results in the answer to question 3, what are your main interventions in this herd?
Table 1. Full differential counts and direct plating from the larger and smaller bulk milk tanks (QMMS Ltd, Wells, Somerset)
| Date Sample Collected: Smaller Tank 2.2.20 9pm Bigger Tank 31.1.20 9pmDate Sample Received: 5 February 2020Date Sample Tested: 5 February 2020Temperature on arrival: 6°C | ||
|---|---|---|
| Sample ID: Smaller Tank 113 cows | ||
| Bulk tank | Count (cfu/ml) | Target (cfu/ml) |
| TBC/TVC (total bacterial count/total viable count) | 78 500 | <5000 |
| Thermoduric count (laboratory pasteurised count) | 445 | <175 |
| Coliform count | 17 | <20 |
| Psychrotrophic count (after 6 days' incubation) | 44 500 | <30 |
| Somatic cell count (,000/ml) | 140 | |
| Direct plating result | Heavy growth of Pseudomonas fluorescens with scant growth of Aerococcus viridans | |
Self assesment: ANSWERS
- Total bacterial count (TBC), also referred to as the total viable count (TVC), measures live bacteria, and counts colony forming units (cfu) on a culture plate, usually at 32–37°C. Bactoscan is a measure of viable and non-viable bacteria and counts individual bacteria rather than colony forming units. The machine counts of ‘impulses’ are then converted to bacterial counts. Bactoscan is always higher than a conventional total bacterial count by a factor of between 5 and 8.
- The main causes of bacteria in milk leading to increased Bactoscan on a dairy farm will be one or more of the following: bacteria from the cow's environment (housed and pastured), including bedding materials and water quality; bacterial pathogens from intramammary infections, most often from missed clinical mastitis events; bacteria present prior to milk harvesting as a result of poor or inadequate teat preparation; bacteria present in the plant resulting from inadequate plant cleaning (wash up routine) and finally bacteria present in the bulk tank itself or from inadequate cooling and refrigeration. A review of Bactoscan failures from 675 herd samples with a TBC >5000 cfu/ml submitted to the QMMS laboratory between 2010 and 2020 was reported by Manning et al (2021). This review showed missed clinical mastitis events resulting in heavy growths of Gram-positive major pathogens (most often Streptococcus spp.) accounted for around one-third of all diagnoses, with poor water quality characterised by the presence of bacteria such as Pseudomonas spp. accounting for around 25% of all diagnoses made. In discussion with your client, you note the following key points:
- Cubicle sticking density at 90% (i.e. 90 cows per 100 cubicles)
- Cubicles and loose yard bedded daily with chopped straw
- Fore-stripping is carried out for clinical mastitis detection
- Pre-milking teat disinfectant used with 30 second contact time before teats are wiped dry with paper towel
- Milking plant wash up routine used water at 90°C (boiler checked and thermometer used) and approximately 20 litres of water per milking unit. Water temperature on circulation is 55–60°C (checked using thermometer)
- Water used for plant cleaning is from mains supply, but water used via the volume washer and for the plate cooler is from a bore hole source and this is not treated prior to use
- Water used through the plate cooler is recycled back into the header tanks used to feed the volume washer
- Only one plate cooler is available (Figure 3)
- TBC and differential counts (thermoduric count, coliform count and psychrotrophic count) are presented in colony forming units per ml (cfu/ml). The ‘smaller’ tank results show the differential count from one milking only and clearly highlight the hugely increased psychrotrophic count, and presence of Pseudomonas fluorescens, both of which suggest the use of a contaminated water source in the parlour. Note that a full psychrotrophic count measures organisms that survive at lower temperatures whereas a thermoduric count measures organisms that survive at higher temperatures, the latter used as a marker for plant cleaning. In this case, the increased thermoduric count is likely to be an artefact of the huge pyschrotrophic count, more than 1000 times higher than the target of less than 30 cfu/ml. The psychrotrophic count and the presence of the Gram-negative, non-coliform organism Pseudomonas spp. is diagnostic of contaminated water sources used as part of the milk harvesting process. The most likely cause for the difference between the ‘smaller’ and ‘bigger’ tank is the time delay between sample collection and testing (psychrotrophic bacteria are more likely to grow at refrigeration temperatures). On this farm, as the larger tank holds milk from three milkings, warmer milk is being added to cold milk from the previous milking and this will bring the overall temperature of the tank up again. As the milk is already ‘fragile’, there will inevitably be some bacterial multiplication, and this is reflected in the vastly increased psychrotrophic count in this sample.
- Water samples were collected from the bore hold source and the header tanks and were submitted to the QMMS laboratory and confirmed an increased total viable count at 22°C (51 cfu/ml for the bore hole supply, >10 000 cfu/ml in the header tank sample) and the presence of Gram-negative environmental organisms were also confirmed in both samples, including Rahnella aquitalis and Aeromonas bestiarum. This confirmed the presumptive diagnosis from the bulk milk samples. The recommendations focused on items relating to water quality in the parlour and as part of the milk harvesting process, and were essentially three-fold:
- UV lamp installed at bore hold water source
- Drain and physically clean out header tanks used to store water for plate cooler and volume washer
- Install new larger plate cooler.

In summary, the increased Bactoscan could be explained by the issues of low-level bore hold water contamination that was then ‘multiplied up’ via a combination of header tanks (roof space, warm, never emptied or cleaned out), recycled plate cooler water (warmed water back into storage which will increase bacterial multiplication) and every other day collection (warm fragile milk added to cold milk and bacterial multiplication able to increase again).
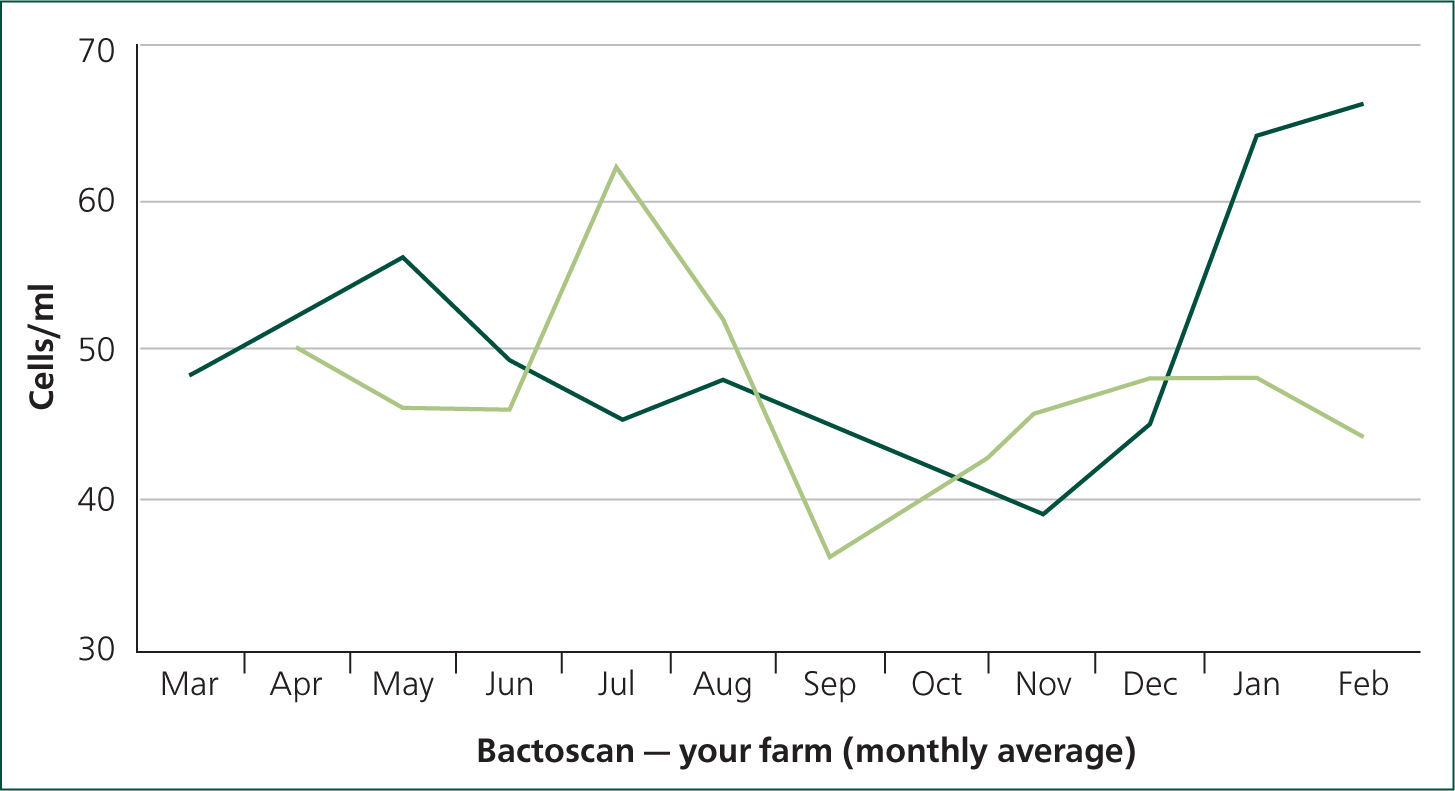
Following the above interventions, the Bactoscan fell dramatically (Figure 4) and is now rolling at 26 impulses/ml for the last 12 months. This self assessment highlights the importance of water quality when considering problems with milk quality on farm.