The global threat of antimicrobial resistance is one that requires united action across different countries and medical disciplines. It is for this reason that numerous European agencies work together to share data and information that can be used to inform policy such as the ‘EU's One Health Action Plan against antimicrobial resistance’. This document outlines multiple strategies that the EU commission plan to use to support member states to ‘preserve the possibility of effective treatment of infections in humans and animals’ (European-Commission, 2017).
One of the many action points within this document is for the European commission to ‘develop EU guidelines for the prudent use of antimicrobials’. One such set of guidelines has been produced by the Antimicrobial Advice Ad Hoc Expert Group (AMEG) who, in 2020, published a categorisation of antibiotics which takes into account the need to use antibiotics in animals versus the risk of antimicrobial resistance to public health. Subsequently an infographic has been developed as a tool to support veterinarians in their decision making when prescribing, which can be found at: https://www.ema.europa.eu/en/documents/report/infographic-categorisation-antibiotics-use-animals-prudent-responsible-use_en.pdf. This tool has been based on the ‘potential consequences to public health of increased antimicrobial resistance when antimicrobials are used in animals and the need for their use in veterinary medicine’ (European Medicines Agency (EMA), 2020). The result is the categorisation of antibiotics into four distinct groups which should be considered every time a veterinarian is administering antibiotics to animals.
The following article describes how this tool can be and has been used in clinical first opinion farm animal practice to help guide responsible prescribing and thus to reduce the overall threat of antimicrobial resistance within animal and human populations.
The Antimicrobial Advice Ad Hoc Expert Group classification
Implementing the AMEG categorisation of antibiotics
The AMEG categorisation of antimicrobials divides all antibiotics that have a license for use in veterinary medicines into four groups which should be consulted whenever a veterinarian prescribes an antibiotic. It should be noted that, as a guideline, this categorisation can and should be considered alongside other influencing factors including constraints on the use of certain antibiotics in food-producing species, information in the ‘summary of product characteristics’ for medicines and regional and local variations in diseases and antibiotic resistance prevalence. The categorisation of antibiotic classes by other stakeholders (e.g. the World Health Organization) may also be relevant in some instances or for a broader context of differing classifications. In the case of farm animal veterinarians, practice policy and farm-specific needs will also likely influence decision making in some instances.
As well as the categorisation of antibiotics, the AMEG guidelines also rank the routes of administration of antibiotics from the lowest to highest estimated impact on antibiotic resistance. Veterinarians are encouraged to consider this ranking when making prescribing decisions. Local, individual treatments (e.g. intramammary preparation or eye drops) should be used where possible as these offer the lowest risk route of administration from an antibiotic resistance perspective. If this is not possible then parenteral individual treatment should be used in preference to oral individual treatments. Injectable group medication in the context of metaphylaxis should only be used when absolutely necessary and should be used in preference to group medication via water/milk which itself should be used in preference to medication in-feed or pre-mixes.
Despite all of the influencing factors that should be considered, it is possible to follow these guidelines in most cases of prescribing to farm species within a practice setting and not compromise on animal health, welfare or client confidence.
Indeed, many veterinarians and practices have long-adopted the principles of responsible antibiotic prescribing. Until recently, and as demonstrated in published case studies, the industry focus has thus been on the reduction and cessation of the use of ‘highest-priority critically important antibiotics’ as defined by the WHO (Allen and Bellini, 2017; Turner et al, 2018). While focus on this aim should not be lost where needed, the publication of this antibiotic categorisation by the EMA can now be used by veterinarians to further scrutinise their prescribing practice and reconsider ‘first-’ and ‘second-line’ antibiotic use.
The four categories of antibiotics
The initial step to utilising the AMEG categorisation in practice is to review the four categories and the guidance on when antibiotics in this category should and should not be prescribed (Figure 1). The four categories to which antibiotics have been allocated are: Category A — Avoid; Category B — Restrict; Category C — Caution; and Category D — Prudence. The next step is to review which antibiotics are in each category and, working through the categories, consider how prescribing from Categories A and B can be reduced in preference for those in Category C and, preferably, D.
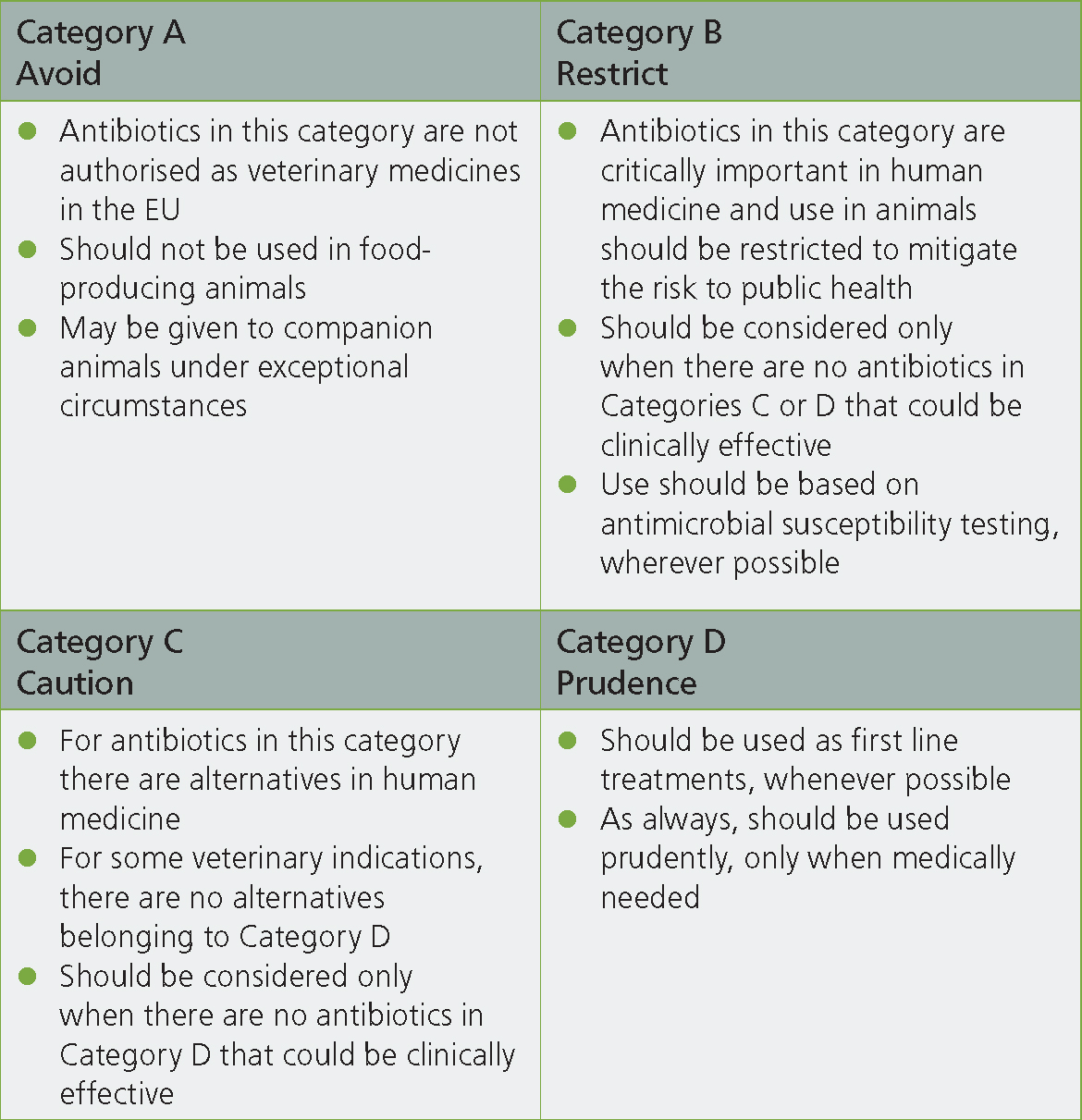
For the farm animal practitioner it should be noted that none of the antibiotics within Category A are licensed in food producing species, and therefore they would be almost impossible to justify under the cascade at least in ruminant species. Given the increasing industry pressures to reduce the use of antibiotics that are important to human health, it is likely that the majority of veterinarians are very familiar with which antibiotics have been placed in Category B — Restrict. These are third- and fourth-generation cephalosporins, fluroquinolones and polymyxins. These antibiotics are already restricted for on-farm use by many stakeholders including the Red Tractor accreditation scheme and many milk buyers. None the less products licenced for use in food producing species are available and can be used following specific justification such as culture and sensitivity results and guidance from a veterinary surgeon.
In the author's experience of teaching final year veterinary students and discussion with veterinarians, the main area of uncertainty seems to surround whether antibiotics fall into Category D (prudence) or Category C (caution), and so it may be that these are the categories that warrant further consideration for prescribing decisions.
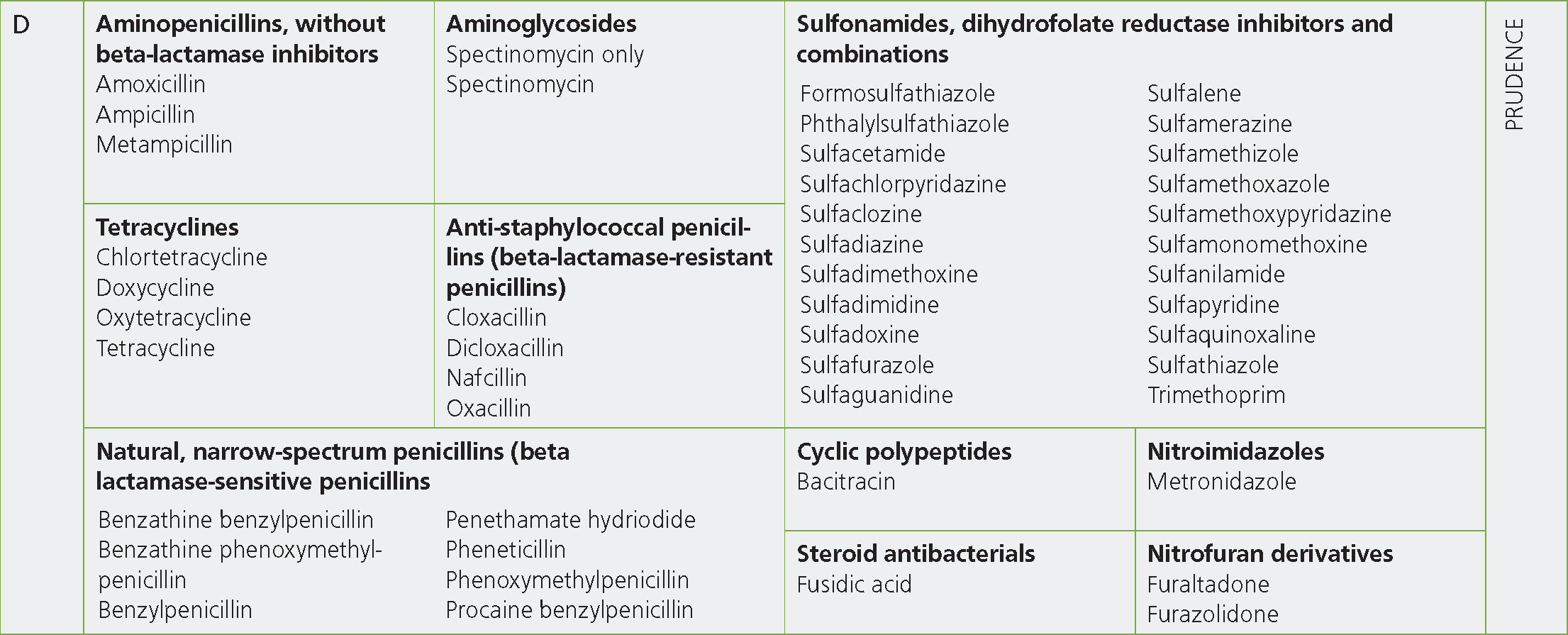
The antibiotics in Category D — Prudence, should be the first option for all clinical situations where empirical prescribing is to be carried out and where there are no prior cases that have had culture and sensitivity results suggesting a Category D antibiotic would be inappropriate (see Box 1). Realistically these cases are likely to account for the majority of prescribing on farms and so should constitute the vast majority of prescribing in the first instance. The Category D antibiotic classes for which there are preparations licensed for food-producing species are: aminopenicillins without beta-lactamase inhibitors, tetracyclines, natural narrow-spectrum penicillins (beta lactamase-sensitive penicillins), anti-staphylococcal penicillins (beta-lactamase-resistant penicillins), aminoglycosides (spectinomycin only), cyclic polypeptides and sulfonamides, dihydrofolate reductase inhibitors and combinations. The category also includes steroid antibacterials, nitrofuran derivatives and nitroimidazoles (including metronidazole which is banned in food-producing animals; Figure 2).
Box 1.A worked exampleAn example of how the AMEG categorisation could be used in practice can be demonstrated by considering the treatment of a 3-week-old calf diagnosed with pneumonia. The initial consideration should be if this is the first case of an outbreak and if any samples to identify the causal pathogen have or should be taken. Assuming this has not yet been done, or the results are not yet known, empirical antibiotic treatment should be considered. Since empirical prescribing is required, Category D antibiotics should be used in the first instance. If there is no evidence to suggest Mycoplasma bovis is involved (ear droop, joint swellings) then either amoxicillin or TMPS are likely to be appropriate for a possible Pasturella multocida infection. If there is evidence of possible M. bovis involvement in this calf or others in the group, and there has not been a culture and sensitivity result to suggest the presence of resistance to tetracycline, then this antibiotic would be a responsible choice. If a previous treatment failure with tetracycline means the presence of resistance is being considered, the dose, route of administration and dosing frequency should be checked, as inappropriate dosing is often a cause of perceived treatment failure. If a culture and sensitivity test from this or a recent outbreak demonstrates that M. bovis with tetracycline resistance is likely to be the cause, then a Category C antibiotic such as florfenicol would be a reasonable choice. It should be noted that a previous tetracycline resistant M. bovis isolate being cultured, possibly years before, does not warrant the ongoing use of Category C or B antibiotics on a farm. Veterinary surgeons should encourage farmers to submit samples from each new outbreak, or at least intermittently to track the presence of pathogens, and resistance patterns on their farms, remembering that resistance prevalence can go down as well as up.
Maximising Category D prescribing
Since these Category D antibiotic classes should be used therapeutically by veterinarians in the first instance where possible, it is worth considering the properties of each as well as the licensing of available products, so as to consider when they can be used to best effect. A full description of the modes of action and efficacy of each antibiotic is beyond the scope of this article, but key features, such as those outlined in Table 1, should always be considered.
Table 1. Pharmacological and clinical information about the Category D antibiotics which should be considered by veterinary surgeons when prescribing, including route of administration for which the products are currently licenced for food producing species in the UK
| Antibiotic class | Spectrum | Mode of action | Comment | Licensed routes (as single component) |
|---|---|---|---|---|
| Penicillins | Gram +ve and anaerobes | Inhibits cell wall synthesis. Bactericidal | Good penetration to most soft tissue. Can penetrate abscesses. No effect against Mycoplasma | IntramuscularLactating cow preparationsOral |
| Amoxicillin | Broad spectrum better action against Gram –ve. less Gram +ve action | Inhibits cell wall synthesis. Bactericidal | Good penetration to most soft tissue. Can penetrate abscesses. No effect against Mycoplasma | IntramuscularDry cow preparationsOral |
| Tetracyclines | Broad spectrum; Gram +ve and –ve, some anaerobes. Active against Mycoplasma and Chlamydia | Inhibits protein synthesis. Bacteriostatic | Good distribution to the urinary tract, respiratory tract, and the intestines | Intravenous IntramuscularOralIntra-uterine Aerosol spray |
| Trimethoprim sulphonamides | Broad spectrum — some anaerobes | Inhibits the folate pathway of bacteria. Bacteriostatic | Inactivated in presence of pus/necrotic tissue. Small molecular size. Pass into inflammatory exudates, cross CSF and placenta. Good distribution in GIT. Not effective against Mycoplasma | Intravenous Intramuscular SubcutaneousOral |
| Aminoglycoside (spectinomycin) | Broad-spectrum, activity against Gram +ve and some Gram –ve bacteria and Mycoplasma, little anaerobic activity | Inhibit protein synthesis. Bactericidal | Largely stays within GIT after oral administration | Oral |
CSF = cerebrospinal fluid; GIT = gastrointestinal tract
The presence of the majority of the ‘first-line ’antibiotic classes in Category D is not surprising to most, but, in the author's personal experience, it is the fact that certain commonly used antibiotic classes are actually in Category C — Caution that is most surprising to veterinarians and veterinary students. In particular the fact that aminopenicillins in combination with beta-lactamase inhibitors (i.e. amoxycillin-clavulanate) are in Category C is surprising to some, and it would seem that the empirical prescription of this combination of antibiotic is not uncommon within livestock veterinary medicine. Penicillin in combination with streptomycin is another combination that is frequently reached for by veterinarians as the broad-spectrum action of the dual combination makes it a popular choice for empirical prescription. However, the inclusion of streptomycin — a Category C aminoglycoside — means that this combination should be used as a ‘second-line’ treatment after, preferably, the narrower-spectrum penicillins, where appropriate, or another of the Category D group.
It should be mentioned at this stage that, although the Category C antibiotics are thought of (and within this article referred to) as ‘second-line’ antibiotics, it is very feasible and preferable that a different Category D antibiotic may be a suitable ‘secondline’ antibiotic choice if a culture and sensitivity result — or even a treatment failure — indicates that antibiotic treatment should be changed. For example, Streptococcus dysgalactiae — a common cause of mastitis in cows and joint ill in lambs — is resistant to tetracycline in up to 90% of cases but is likely to respond to penicillin as no isolates have been found to be resistant to this antibiotic (Veterinary Medicines Directorate, 2021). Hence, successful treatment might be achieved without the need to prescribe a category C antibiotic at all.
Reducing prescribing of Category C and B antibiotics
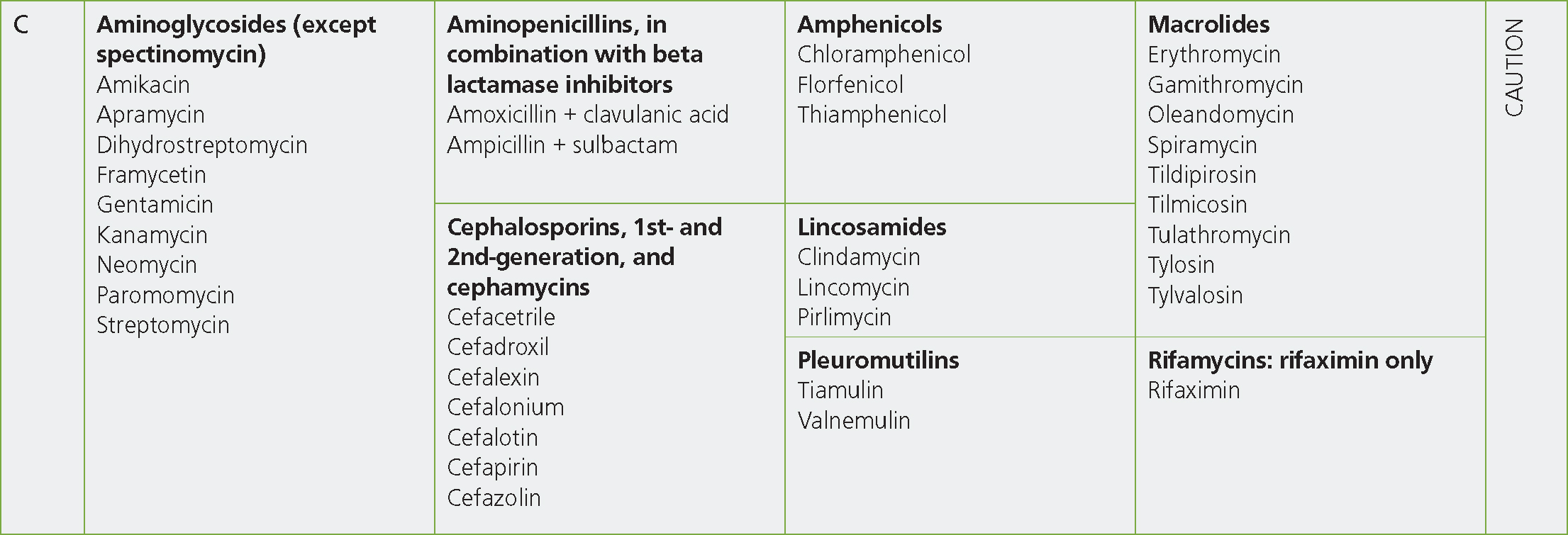
As well as aminoglycosides (except spectinomycin) and aminopenicillins in combination with beta-lactamase inhibitors, the other antibiotic classes in Category C include the first- and second-generation cephalosporins, amphenicols, lincosamides, pleuromutilins, macrolides and rifamixin (Figure 3). Given the broad range of antibiotic classes included in Category C and the various modes of action and features of these antibiotics there are (in the author's opinion) often multiple ‘second’-line' treatment options within this group that can be utilised if there are no Category D antibiotics that are suitable or to which the isolate is sensitive. This means the use of Category B antibiotics should very rarely, if ever, be necessary. The prescription of Category C antibiotics, particularly for empirical and ‘first-line’ treatments should, however, be reviewed and Category D antibiotics utilised in their place wherever possible. Practitioners may be concerned about the prescription of Category D antibiotics because of resistance to these classes, however when testing is being performed there is very often an option for the prescription of a Category D antibiotic even if resistance to another is common. For example, Pasteurella multocida isolated from cattle is frequently resistant to tetracyclines (47%), however, in a recent report only 1.4% of these isolates were resistant to ampicillin or to trimethoprim sulphonamides (Veterinary Medicines Directorate, 2021). A higher percentage of P. multocida isolates from pigs were resistant to trimethoprim sulphonamides (17%), but tetracycline resistance was lower than in cattle isolates (20%) (Veterinary Medicines Directorate, 2021). This demonstrates that in cases where resistance to Category D antibiotics is known to be common, culture and sensitivity testing is prudent to select the most appropriate treatment choice and is more responsible than the empirical prescription of Category C or even Category B antibiotic classes.
Category B includes the third- and fourth-generation cephalosporins, the fluroquinolones and polymyxins (Figure 4). These classes of antibiotics have also been classified by the World Health Organization as ‘highest-priority critically important antimicrobials’. As has been demonstrated by many veterinarians and practices already, the reduction and cessation of the use of these classes of antibiotics in practice is certainly achievable (Allen and Bellini, 2017; Turner et al, 2018).
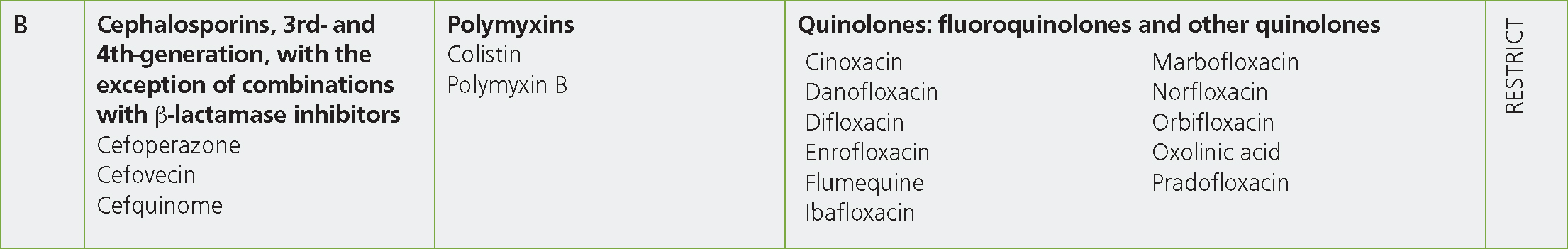
The use of parenteral third-generation cephalosporins can be replaced with first-generation cephalosporins (Category C — Caution) if zero milk withdrawal is an important feature of treatment or with a Category D antibiotic class in the majority of cases. The only fourth-generation cephalosporin in licensed products for food-producing species is cefquinome. This antibiotic is available in the form of intramammary suspensions (for both dry cow and lactating cow therapies) and as a parenteral injection for the treatment of susceptible organisms in cattle and pigs. Replacing its intramammary tube use with antibiotics from Category C is relatively straight forward as there are numerous efficacious lactating and dry cow tubes on the market that contain Category C antibiotics. Replacing its use with Category D antibiotics, particularly for lactating cow therapies, may be more difficult as the lactating cow preparations containing Category D antibiotics that are available offer only narrower spectrum activity, and so may not be suitable in all cases. A further consideration for the clinician is the use of intramammary tubes that contain multiple antibiotic products. As per responsible antimicrobial use guidelines, narrow-spectrum products should be used in favour of broad-spectrum products and, where possible, single-product tubes would be preferable to those containing multiple antibiotic classes (European Union, 2015). In either case, and as also highlighted in the AMEG guideline, intramammary products are preferable to injectable products for the treatment of local, intramammary infections. While not included in the AMEG guidelines, prescribing veterinary surgeons may also consider the use of ‘dual therapies’ (ie: simultaneous dosing with injectable and intramammary preparations) for the treatment of some conditions, commonly mastitis. It should be highlighted that the evidence for such dual dosing is scarce for the majority of mastitis pathogens (Ruegg, 2021), other than for the treatment of Staphylococcus aureus where benefits have been shown (Barkema et al, 2006).
The potential uses of fluroquinolones in farm animal practice are wide and varied, and for this reason they may be replaced with a number of different Category D antibiotics, dependent on the clinical situation. As already described, Category D antibiotic classes are likely to offer successful treatments for the vast majority of clinical scenarios on farms — even in cases where resistance is common — if culture and sensitivity, or reference to resistance reporting (for example the VARSS reports), can be used to guide treatment.
The use of colistin in veterinary medicine has historically been primarily for group-dosing of pigs and poultry against Gram-negative infections. The use of all antibiotics has reduced significantly in these sectors in recent years, with specific attention being paid to Category B antibiotics. The British Poultry Council reported the complete cessation of the use of colistin by its members by 2018 and no products containing colistin were reported to have been used in pigs in the UK in the 2020 VARSS report (Veterinary Medicines Directorate, 2021).
Utilising testing to improve prescribing decisions
While the focus of this article has been empirical prescribing of antibiotics it should be highlighted that culture and sensitivity testing is, in general, hugely under-utilised in farm animal practice and should be used more to encourage, or confirm the responsible prescription of antibiotics. Culture and sensitivity testing is by no means required in every case that antibiotics are to be prescribed, but sampling and testing at the start of an outbreak situation, when new disease patterns are recognised or even intermittently in the case of endemic disease, would assist in identifying the most likely pathogens and susceptibilities to antibiotic treatments. It is likely that such testing would not only identify where Category D antibiotics could be a suitable choice, but also increase the confidence of the veterinary surgeon and the farmer in these treatment choices. Veterinary surgeons are increasingly being challenged to use antibiotics more responsibly, and so it should be questioned how we can do this if, most of the time, we do not know what bacteria we are treating or what antibiotic treatment that bacteria will respond to?
KEY POINTS
- The route of administration of antibiotics can affect their potential to cause antibiotic resistance.
- Antibiotics from Category D should be used for all empirical prescribing unless culture and sensitivity testing suggests this would be inappropriate.
- A different Category D antibiotic may be appropriate as a ‘second line’ treatment choice in some instances.
- Category C antibiotics should only be used where Category D antibiotics are inappropriate or not licenced for a particular use.
- The use of all Category B antibiotics should be reviewed and ceased in preference for category D or C where possible.
- Culture and sensitivity testing is underutilised and should be encouraged by veterinary surgeons and done routinely and regularly.
Conclusion
As has been demonstrated by multiple veterinarians and veterinary practices across the UK, moving towards ever more responsible antibiotic prescribing in line with the AMEG guidelines is certainly possible. In fact, in many cases, and in response to various pressures, veterinarians, veterinary practices, farms and farming sectors have ceased or significantly reduced their prescription of Category B antibiotics. For those still prescribing Category B antibiotics it is clear that a precedent has been set and now more than ever the use of these antibiotics should be ceased. Beyond the cessation or reduction of Category B antibiotics it is now time to use the AMEG guidance tool to focus our attention on further moving towards more responsible prescribing by reviewing the use of Category C antibiotics and, where possible, using Category D antibiotics for the majority, if not all, of empirical or first-line treatment choices alongside increased utilisation of culture and sensitivity testing to inform best prescribing practice.