In dairy production, fertility is one of the main drivers of herd profitability through its impact on achieving greater production and maintaining short calving intervals. This is particularly true of seasonal systems with short, well-defined breeding periods where calving pattern (driven by submission rate and conception rate) is a key driver of profitability. Reproductive efficiency is also critical to the sustainability of suckler beef production. In systems where the calf is the only output (apart from cull cow sales), achieving a calf per cow per year is an important goal. However, reproductive efficiency in many beef cow herds is suboptimal. Despite the role that suckler beef production plays in many rural communities, its future sustainability is under threat, because of poor profitability in the sector and also from climate change, with recent calls to reduce the national herd significantly in an effort to curb greenhouse gas emissions.
Successful pregnancy establishment involves ovulation of a competent oocyte, fertilisation by a capacitated sperm and growth of the embryo in a reproductive tract environment conducive to normal development. Following fertilisation in the oviduct, the bovine embryo enters the uterus at about the 16-cell stage on approximately day 4 of pregnancy. By day 7, the embryo has formed a blastocyst consisting of an inner cell mass, which, after further differentiation, gives rise to the embryo/fetus, and the trophectoderm, which ultimately forms the placenta. After hatching from the zona pellucida on day 9 to 10, the spherical blastocyst undergoes a change in morphology from a spherical to ovoid shape during a transitory phase preceding the elongation or outgrowth of the trophectoderm to a filamentous form that usually begins between day 12 and 14. Around this time, the trophectoderm cells of the conceptus begin to secrete significant amounts of interferon-tau, the pregnancy recognition factor in cattle, which ultimately block the uterine luteolytic mechanism to ensure maintenance of a functional corpus luteum for the production of progesterone (P4). This is a critical period of development as most embryonic loss occurs during this window (Wiltbank et al, 2016; Berg et al, 2022).
While fertilisation success is typically high (>85%) following artificial insemination (AI), birth rates are significantly lower, indicating the occurrence of embryonic death and fetal losses during pregnancy. A significant proportion of this loss occurs between fertilisation and maternal recognition of pregnancy, which in cattle occurs around day 16 post-oestrus; indeed, in high-producing dairy cows, as much as 50% of embryos may be no longer viable by day 7 (Wiltbank et al, 2016; Berg et al, 2022). These losses are likely due to deficiencies in oocyte quality and early embryo development, with additional losses attributed to uterine dysfunction or failure of the conceptus to develop appropriately, signal pregnancy recognition and/or undergo attachment, implantation and placentation.
The success of in vitro fertilisation (IVF), where embryos are made in the laboratory, demonstrates that contact with the female reproductive tract is not necessary in order for the embryo to reach the hatched blastocyst stage. However, the characteristic elongation of the ruminant conceptus prior to implantation is dependent on secretions from the uterus, as evidenced by the fact that it does not occur in vitro or in vivo in the absence of uterine glands (Gray et al, 2002). This highlights the key role played by the uterine endometrium in driving the elongation process via endometrial secretions which make up the uterine lumen fluid. Temporal changes of the endometrial gene expression pattern (transcriptome) and uterine lumen fluid composition are necessary to establish uterine receptivity to implantation and, in turn, are pivotal to the success of pregnancy establishment. These modifications are regulated by conceptus-derived interferon-tau together with maternally-derived P4 from the corpus luteum, to induce expression of genes in uterine luminal and superficial glandular epithelia for transport and/or secretion into the uterine lumen to support growth and development of the conceptus.
Circulating concentrations of P4 represent a balance between production and metabolism of P4 (Wiltbank et al, 2014). Elevated P4 during growth of the preovulatory follicle have been shown to increase pregnancy per AI. Low circulating concentrations of P4 near AI, indicative of optimal corpus luteum regression, are required to optimise fertility, while elevated P4 concentrations after AI can impact embryonic development and may, in some circumstances, improve fertility. Thus, strategies to optimise P4 concentrations during selected reproductive periods may improve reproductive efficiency of lactating dairy cows (Wiltbank et al, 2014).
The aim of this article is to highlight some recent findings in relation to embryo maternal interaction during pregnancy establishment in cattle and the role of P4 in in uterine biology and embryo development. For a more comprehensive treatment, the reader is referred to some of the numerous detailed reviews listed in the reference section (eg Inskeep, 2004; Wiltbank et al, 2014; Spencer et al, 2016; Lonergan and Sánchez, 2020).
P4 and the oocyte
High P4 concentrations during the growth of the ovulatory follicle are associated with improved oocyte quality and pregnancy outcomes (Pursley and Martins, 2012; Wiltbank et al, 2014; Bisinotto et al, 2015). During the final period of follicle growth, between the preovulatory luteinising hormone surge and ovulation, the follicular fluid changes from an environment dominated by oestradiol to one that is dominated by P4 (Dieleman et al, 1983) as the granulosa cells luteinise in preparation for the formation of the corpus luteum after ovulation. Given that this is coincident with resumption of meiosis and maturation of the oocyte, a role in determining oocyte quality is likely. Inhibition of P4 synthesis by cumulus cells in vitro resulted in a decrease in bovine embryo development, indicating that P4 intracellular signalling is mediated by its interaction with nuclear and membrane P4 receptors and is important for oocyte developmental competence (Aparicio et al, 2011).
Reduced P4 concentrations, as seen during growth of the first follicular wave when the corpus luteum is still developing, negatively affect embryo quality after super stimulation (Rivera et al, 2011; Nasser et al, 2011) and reduces pregnancies per AI of lactating dairy cows (Denicol et al, 2012). In both scenarios, outcomes were improved by supplementing with exogenous P4 via an intravaginal device. Based on such studies, sophisticated hormonal synchronisation protocols have been incorporated widely into reproductive management programs, many of which use a P4 device (Carvalho et al, 2018) or other strategy to ensure elevated P4, such as induction of an accessory corpus luteum.
Intravaginal P4 devices are one of the main delivery methods for exogenous P4 during oestrous synchronization protocols. A study by van Werven et al (2013) compared circulating P4 concentrations and pregnancies per AI in dairy cows following oestrous synchronisation with one of two commercially available and commonly used intravaginal P4 devices. PRID-Delta produced significantly greater circulating P4 peak compared to a controlled internal drug release dispenser (CIDR). In addition, P4 concentrations were greater for PRID-Delta starting on the same day of device insertion until day 4. PRID-Delta tended (P=0.10) to produce greater pregnancies per AI at first AI when compared to CIDR, which may be related to greater circulating P4 during the synchronisation program. Furthermore, it has been recently reported that enhanced P4 support during follicle-stimulating hormonestimulated cycles of transvaginal follicular aspiration, through the insertion of an intravaginal P4 device, improves bovine embryo production in vitro, the effect being greatest with the PRID-Delta (Simmons et al, 2023).
P4 and the uterus
The relationship between circulating P4 and uterine receptivity has been well described (Spencer et al, 2016; Lonergan and Sánchez, 2020). Elevated P4 concentrations in the first week after conception have been associated with accelerated post-hatching conceptus elongation, mediated through advancement in the regular temporal changes in the uterine endometrial transcriptome (Forde et al, 2009) and alterations in the uterine lumen fluid composition (Simintiras et al, 2019).
P4 priming of the uterus is essential for optimal pregnancy establishment. As the corpus luteum develops following ovulation, the uterus is exposed to increasing concentrations of P4, which alter the gene expression pattern (transcriptome) of the endometrium. By comparing the transcriptome of cyclic and pregnant bovine endometrium, it is clear that temporal changes in endometrial gene expression occur irrespective of whether the cow is pregnant or not and it is really only at the time of maternal recognition of pregnancy at around day 16 that major changes in gene expression are detectable between pregnant and cyclic animals (Forde et al, 2011a). An adequate rise in P4 after ovulation drives these normal temporal changes that occur in the endometrial transcriptome of cattle that are necessary for the establishment of uterine receptivity and the promotion of conceptus development. Forde et al (2009) described the global transcriptome of the endometrium from day 5–16 in pregnant and cyclic cattle under conditions of normal and elevated P4, revealing how circulating concentrations of P4 regulate endometrial genes. The study found that P4 supplementation advances the normal temporal changes in endometrial gene expression, particularly for genes associated with energy sources or contributors to histotroph, which may contribute to advanced conceptus development on day 13 and day 16. In contrast, low P4 was associated with an altered endometrial transcriptome and retarded conceptus elongation (Forde et al, 2011b; 2012). The embryo does not have to be present in the uterus during the period of P4 elevation in order to benefit from it (Clemente et al, 2009), supporting the concept that the positive effect on conceptus growth is mediated via P4-induced changes in the endometrial transcriptome.
Conceptus elongation, which occurs during the second week of development in cattle, is a maternally driven process – despite the ability to produce large numbers of blastocyst stage embryos using IVF, post-hatching elongation has not been recapitulated in the laboratory nor does not it occur in recipients experimentally induced to have no glands (Gray et al, 2002). Interestingly, elongation also appears to be associated with oocyte quality, as has been observed over numerous studies that in vitro produced blastocysts transferred in groups to the same uterus elongate at different rates (Figure 1). This is important because short (retarded) conceptuses have a different gene expression pattern to their longer age-matched counterparts (Barnwell et al, 2016; Ribeiro et al, 2016) and such short conceptuses produce less interferon-tau and fail to elicit an appropriate response from the endometrium around the time of pregnancy recognition (Sánchez et al, 2019).
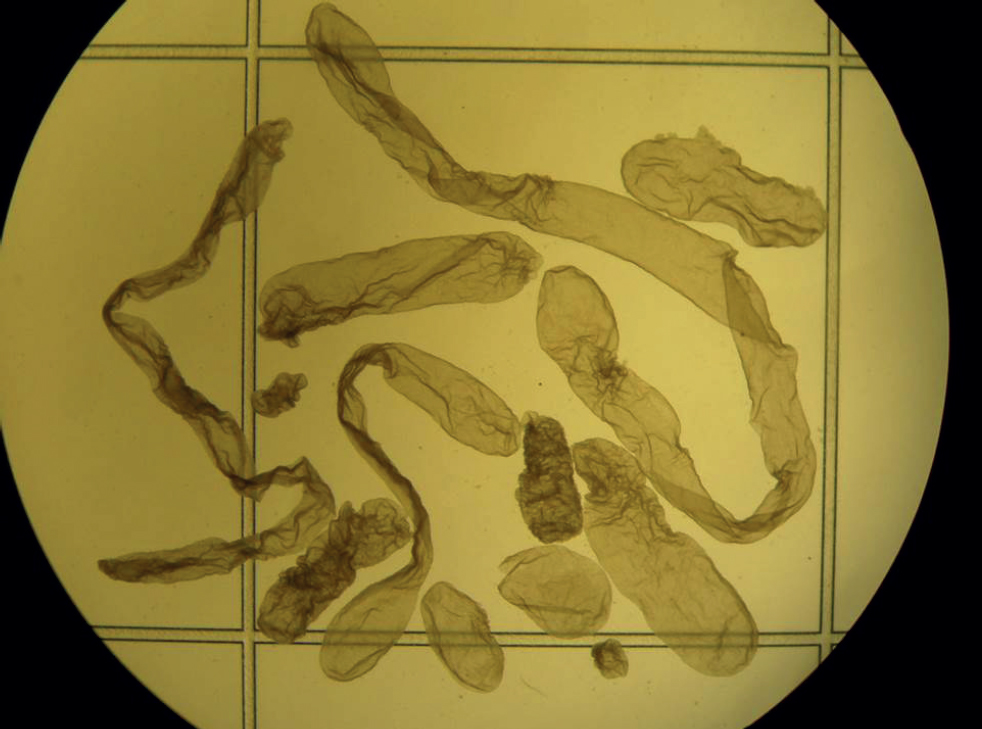
P4 and embryo transfer success
‘Beef on dairy’ is currently a hot topic. While a small proportion of male dairy calves (around 0.1%) are genetically elite and of value as potential future AI bulls, the majority have low economic value because of their poor future beef value and present welfare, social and environmental concerns. As a consequence, the use of both sex-sorted dairy semen (to generate replacement females) and conventional beef semen (to generate all remaining pregnancies) is increasing, facilitating genetic gain in replacement stock while enhancing the beef value of surplus calves (Crowe et al, 2021a). Although beef-cross calves have greater economic value than male dairy calves, further gains are potentially feasible through the transfer of purebred beef embryos.
According to the latest data available from the International Embryo Technology Society (www.iets.org), a new milestone was reached in 2021 with over one million transfers of in vitro produced embryos worldwide (Viana, 2022). Since 2017, the number of in vitro produced embryos transferred has surpassed the number derived by traditional superovulation (Crowe et al, 2021b) accounting for approximately 80% of all bovine embryos produced and transferred in 2021 (Viana, 2022). Although issues with cryotolerance (ie freezability) of in vitro produced embryos, embryo loss, and, in some cases, calf birth weight remain to be fully resolved, in vitro production embryos are likely here to stay as a tool for genetic improvement in dairy herds (Sanches et al, 2019), offering increased flexibility in sire usage allowing multiple pregnancies from elite dam-bull combinations to be generated and the ability to produce more embryos per unit time than traditional superovulation, which involves stimulation of the female with follicle stimulating hormone to cause multiple ovulations, followed by AI and non-surgical embryo recovery 7 days later.
To test the feasibility of using IVF in our seasonal pasture-based system of production, the author recently carried out a large-scale field trial to examine fertility in lactating dairy cows following timed AI or timed embryo transfer with fresh or frozen in vitro produced embryos (Crowe et al, 2023). Pregnancy rates for freshly transferred embryos were comparable with those achieved after AI. However, consistent with other studies, embryonic loss between day 32 and day 62 was increased with in vitro produced embryos compared to AI, particularly when frozen embryos were transferred.
Among other parameters, serum P4 concentration was measured on the day of embryo transfer and retrospectively correlated with pregnancy rate. Among cows deemed to be suitable for embryo transfer based on presence and size of the corpus luteum on the day of transfer, those with greater serum concentrations of P4 on day 7 had greater pregnancy per service event at both day 32 and day 62 (Table 1; Figure 2). Irrespective of service type (AI or embryo transfer), cows in quartile 1 (ie least P4 concentration) had lesser probability (P<0.0001) of being pregnant on day 32 (33.4%) and day 62 (30.1%) than cows in quartile 2 (day 32 = 45.7%; day 62 = 42.2%), quartile 3 (day 32 = 55.6%; day 62 = 49.2%) or quartile 4 (day 32 = 61.2%; day 62 = 54.6%). Cows in quartile 2 had similar probability of pregnancy (P=0.102) as cows in quartile 3, and had lesser probability of becoming pregnant than cows in quartile 4 (P=0.002).
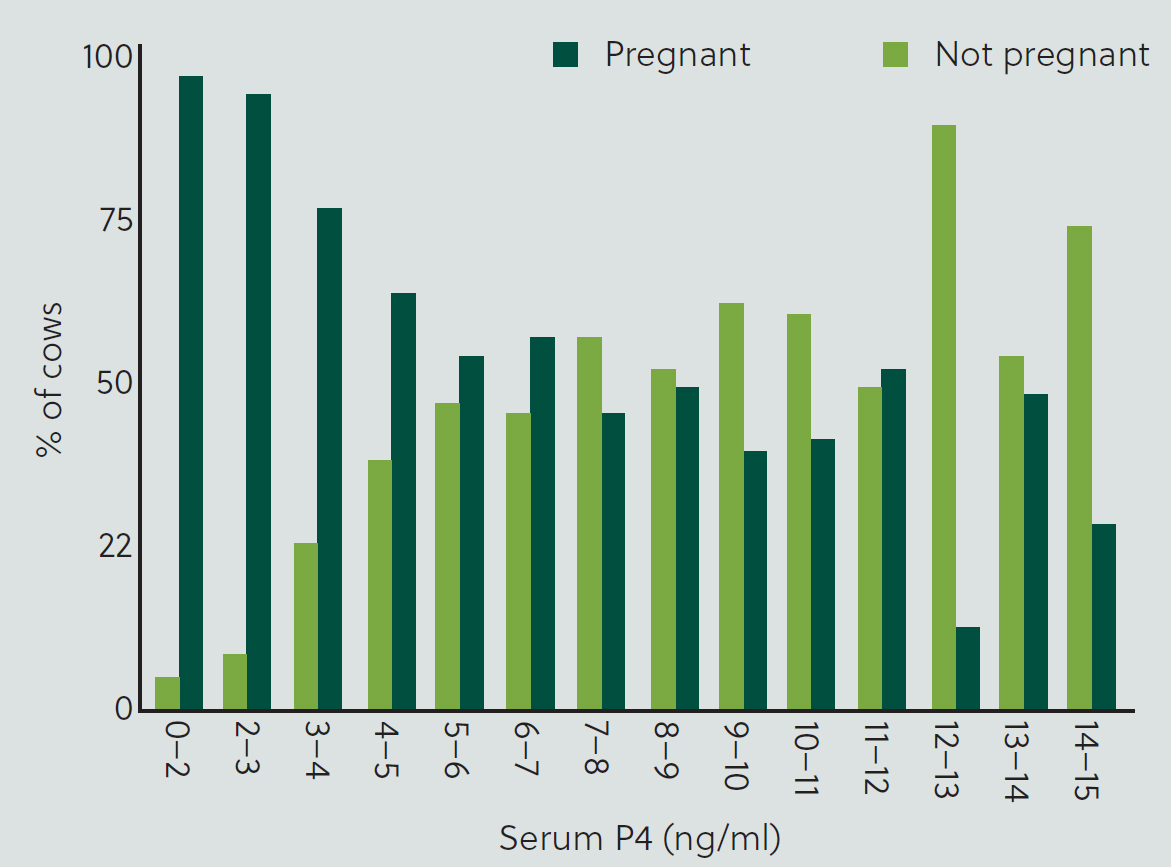
Table 1. Mean pregnancy/service event (P/S) in lactating dairy cows based on quartiles of serum progesterone (P4) concentration on the day of embryo transfer
| Quartile | P4 ng/ml | n | P/S day 32 | P/S day 62 |
|---|---|---|---|---|
| Mean | Mean | |||
| 1 | <5.79 | 274 | 33.4%a (27.7–39.7) | 30.1%a (24.5–36.4) |
| 2 | 5.79–7.36 | 274 | 45.7%b (39.1–52.4) | 42.2%b (35.7–49.0) |
| 3 | 7.37–9.42 | 274 | 55.6%bc (49.1–61.9) | 49.2%bc (42.8–55.7) |
| 4 | >9.42 | 275 | 61.2%c (54.7–67.4) | 54.6%c (48.0–61.1) |
a-cMean values in the same column with different superscripts differ (P<0.025). Values in brackets represent 95% confidence intervals. Modified from Crowe et al (2023).
Consistent with other studies, there was a positive, quadratic relationship between serum P4 on day 7 and likelihood of pregnancy establishment, irrespective of method of breeding (AI or embryo transfer); cows with P4 concentrations in the quartile with the least P4 concentrations were almost half as likely to become pregnant than those in the upper quartile. Wallace et al (2011) reported that administration of human chorionic gonadotropin (hCG) at the same time as embryo transfer increased incidence of accessory corpus luteum formation, serum P4 in pregnant recipients, and P/embryo transfer and reduced early embryonic losses after transfer. Treatment of cows with gonadotropin hormonereleasing hormone (GnRH) on day 5 after oestrus to induce an accessory corpus luteum and elevated P4 was reported to also reduce embryo loss between day 33 and day 60 in heifers following transfer of in vitro produced embryos (García-Guerra et al, 2020).
P4 supplementation studies
Many studies have attempted to improve fertility in cattle by elevating P4 after AI (Lonergan and Sánchez, 2020). Approaches taken to increase peripheral concentrations of P4 after AI include those that:
- Increase function of the existing corpus luteum (eg promote growth of the dominant follicle before ovulation, resulting in a larger corpus luteum, or luteotrophic treatments which stimulate corpus luteum development such as hCG administration)
- Induce ovulation of a dominant follicle and formation of accessory corpus luteum (eg hCG or GnRH administration)
- Or those which supplement progesterone directly (eg via injection or intravaginal devices).
Data on outcome in terms of pregnancy rate are often conflicting or inconclusive. Thus, while a significant volume of research has provided insight into the mechanisms regulating circulating P4 concentrations and actions on the uterus and conceptus, more research is required to best understand how P4 manipulation can be repeatedly used to improve reproductive success.
Conclusion
Low P4 concentrations have been implicated as a causative factor in low pregnancy rates observed in high-yielding dairy cows. Optimal P4 during growth of the ovulatory follicle results in improved outcomes, presumably through effects on the quality of the ovulated oocyte. While effects of P4 on the early pre-hatched embryo have not been convincingly demonstrated, elevated concentrations of P4 in the immediate post-conception period have been associated with an advancement of conceptus elongation, an increase in interferon-tau production and higher pregnancy rates in cattle. Elevated P4 advances the transcriptomic changes in the endometrium which normally occur during pregnancy, resulting in enhanced conceptus elongation. The embryo does not have to be present in the uterus during the period of P4 elevation in order to benefit from it, supporting the concept that the positive effect on conceptus growth is mediated via P4-induced changes in the endometrial transcriptome. Innovative strategies aimed at optimising circulating concentrations of P4 pre- and post-conception have the potential to improve embryo survival in cattle.
Future studies
Emerging data indicate that the time of presumptive conceptus attachment (based on initial increase in pregnancy-specific protein B (PSPB) following AI) is critical for pregnancy success (Middleton et al, 2019; Santos et al, 2023). Cows in which presumptive conceptus attachment occurred on or after day 23 had an 80% likelihood of losing the pregnancy compared to those that attached earlier (Santos et al, 2023). Whether later attachment could partly explain the increased loss following the transfer of in vitro produced embryos and whether elevated P4, which accelerates conceptus elongation, is associated with earlier conceptus attachment is an exciting area that remains to be fully tested.
KEY POINTS
- Successful growth and development of the post-hatching blastocyst and pregnancy establishment are a result of the interaction between a competent embryo and a receptive uterine environment.
- Progesterone (P4) plays a key role in reproductive events associated with the establishment and maintenance of pregnancy, mainly through its action on the uterine endometrium.
- Low concentrations of circulating P4 after ovulation are associated with a reduction in conceptus growth and elongation, a decrease in interferon-tau production and lower pregnancy rates in cattle.
- Elevated concentrations of circulating P4 in the immediate post-conception period are associated with an acceleration of conceptus elongation, an increase in interferon-tau production and, in some cases, higher pregnancy rates.
- Despite the potential beneficial effects of exogenous P4 supplementation on fertility, results of supplementation studies have been inconsistent and may be related to the strategy used to elevate P4, the type of animal (eg lactating or not), as well as endogenous concentrations in the animal.


