









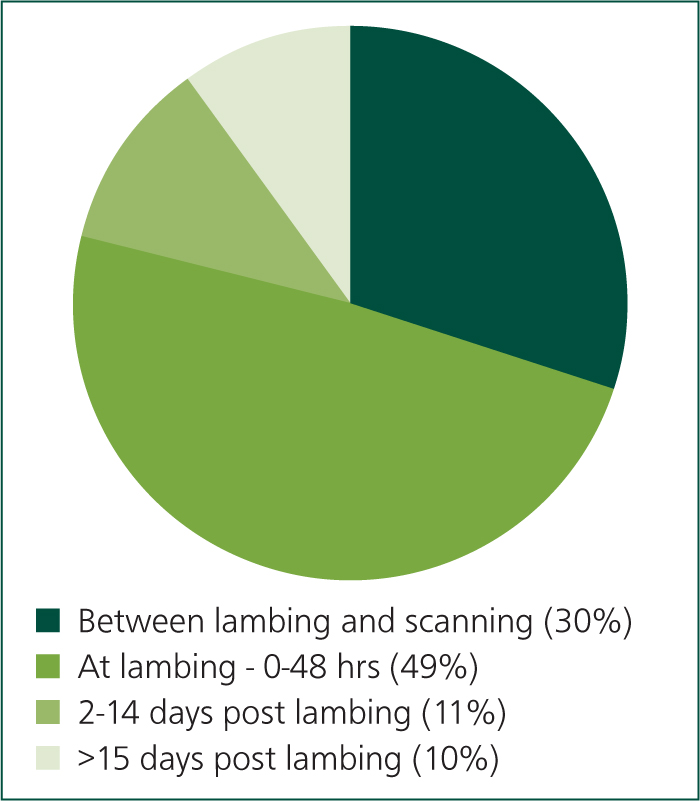
Losses associated with ovine abortion have a significant impact on the profitability of sheep farms. Further losses, with the same aetiologies as the abortions, occur as a result of weak, illthrift lambs that are born alive but may die or require the input of time and expense to survive. Lamb growth rates are impacted, further reducing profitability. Abortion has a negative impact on the health and welfare of both the flock and those working with it. Many of the agents that cause abortion have the potential to cause serious human physical illness, and losses at lambing can have a significant impact on the mental health of those working with the flock. The annual incidence of abortion is estimated to be 2–3% (Mearns, 2007a) and abortion accounts for 30% of total lamb losses (Figure 1, Hybu Cig Cymru (HCC) lambing project). The annual cost of chlamydial abortion alone to the UK sheep industry has been estimated to be £11–48 million per year (Bennett and IJpelaar, 2005) and the total cost from all causes of abortion collectively can reasonably be expected to be more than double these figures.
Surveillance for ovine abortion
The Animal and Plant Health Agency (APHA) performs surveillance to monitor the health of the UK's breeding flock in order to detect undefined or unexpected trends and to enable timely mitigation of potential impacts on public health, animal welfare, livestock productivity, the environment and wider society. This happens through the ongoing systematic collection, analysis and interpretation of data and the dissemination of information to the sheep sector. The APHA take particular interest in the identification of novel diseases or pathogens, exotic or notifiable diseases, emergence of new pathogen variants/subtypes and in toxic or zoonotic threats to public health. Where there is a significant threat to a population, such as the threat of abortion in sheep, the APHA pay attention to changes in endemic disease trends. Ovine abortion is also important in the context of human health with enzootic abortion in ewes (EAE), Toxoplasma gondii, Campylobacter spp., Coxiella burnetii (Q fever), Salmonella spp., Listeria monocytogenes and Listeria ivanovii, leptospirosis and brucellosis all being zoonotic. Brucellosis is notifiable, Salmonella spp. and Q fever caused by Coxiella burnetti are reportable.
In order to achieve its objectives the APHA has a number of veterinary investigation centres (VIC) in England and Wales (Thirsk, Penrith, Shrewsbury, Bury St Edmunds, Starcross and Carmarthen) and works with the disease surveillance centres at the Scottish Rural College (SRUC) in Scotland (Aberdeen, Ayr, Dumfries, Edinburgh, Inverness, Perth, St Boswell's and Thurso) and the SRUC post-mortem examination service at the University of Glasgow. In addition, the APHA has contracted providers at the Universities of Surrey, Bristol, Liverpool, Nottingham, the Royal Veterinary College, and Cambridge, and the Wales Veterinary Science Centre. Farmers that are further than 1 hour from any centre also benefit from a free carcass collection service.
A standardised submission form is used with samples that are received and this submission information is important in providing baseline data that allow the determination of disease incidence. Standardised data from submission forms are collated within the APHA database which contains details of all diagnoses back to 1975. Algorithms are run on the data quarterly in order to identify statistically significant trends with seven diagnostic codes for ovine abortion being monitored specifically.
Where specific issues are identified on farm, the APHA will carry out farm visits to further investigate. Farmers whose sheep have suffered infectious causes of abortion are sent reminders in June to encourage them to consider vaccination the following year. Results of surveillance are communicated to the veterinary profession and to sheep farmers through a number of channels including quarterly surveillance reports, monthly updates in the Veterinary Record, focus articles in the Veterinary Record, contributions to sheep farmer magazine, the small ruminant pages of the APHA's Vet Gateway and Facebook.
Causes of ovine abortion in the UK
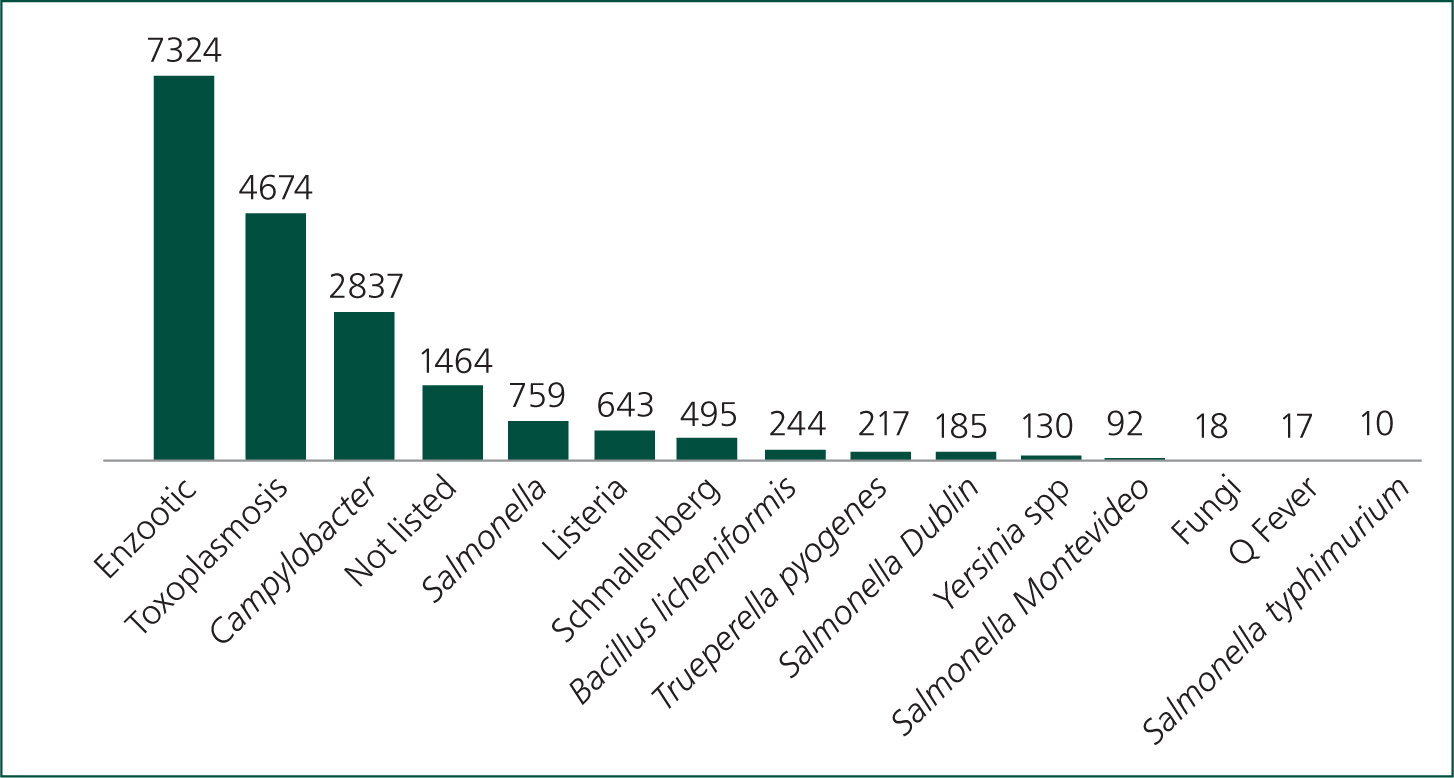
The frequency of diagnosis of ovine abortion at the APHA between 2010 and 2021 is shown in Figure 2. Trends are consistent year on year. EAE is consistently the most frequent diagnosis followed in descending order by infection with Toxoplasma gondii, Campylobacter spp., Salmonella spp. and Listeria spp.
Chlamydia abortus (enzootic abortion in ewes)
C. abortus (formerly C. psittaci) is a Gram-negative bacterium and is the most common cause of abortion in sheep in the UK. Some areas of the Highlands and Islands of Scotland are free of the disease and a number of flocks are warranting that they are free of the disease through EAE accreditation schemes. Infection with C. abortus occurs orally and is asymptomatic until the next pregnancy when the organism invades the placenta around 90 days' gestation, creating a suppurative necrotising placentitis resulting in abortion or retardation of fetal growth (Figure 3) (Mearns, 2007a; Essig and Longbottom, 2015; OIE, 2018a). If infection occurs more than 6 weeks before the due date then it is possible for infection and abortion to occur within the same pregnancy; however, infection typically occurs one year and abortion the next. While infection predominantly occurs through contact with aborted material there are field studies that have demonstrated horizontal transmission between lambing seasons with some ewes thought to excrete the infectious organism at oestrus in vaginal fluids and in faeces (Papp et al, 1994; Papp and Shewen, 1996; Winter et al, 2002; Livingstone et al, 2009). Although venereal infection is thought to occur uncommonly, if at all, rams can be infected and develop orchitis.
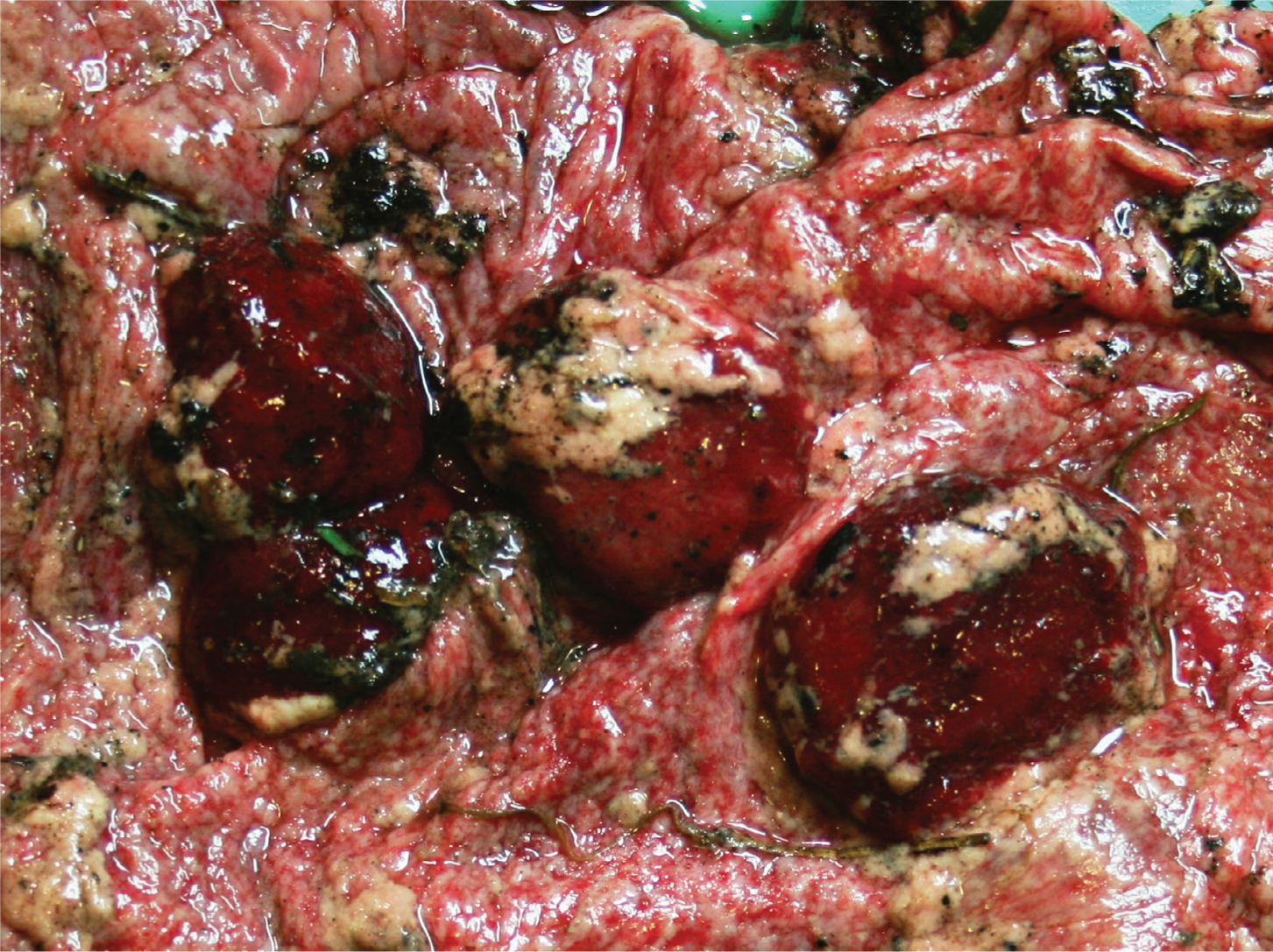
In flocks where EAE is endemic, and there is some degree of immunity among previously aborted animals, abortion rates of 5–10% are common. In naïve populations, introduction usually results in a small number of abortions in the first year, with an abortion storm with over 30% of ewes aborting the following year (Essig and Longbottom, 2015). Abortion rates above 40% have been seen in particularly severe outbreaks (JP Crilly, personal observation). Even ewes that do not abort may produce weak lambs that subsequently die. Infected ewes are generally well in themselves, although they may show lethargy for 24 hours but do not develop signs of systemic illness. Abortion occurs in the last 3 weeks of gestation and the placenta is typically covered in a brown exudate and has thickened areas between the cotyledons. Lambs may be dead or alive (and potentially both from the same pregnancy), with dead lambs having a normal appearance. The aborted material and subsequent vaginal discharges will be heavily contaminated with the infectious organism for up to 3 weeks, which will then remain viable in the environment for up to 6 weeks (Carson et al, 2019). After aborting, the vast majority of ewes are immune and will not abort again. A small number will remain infected and can shed at subsequent lambings, and at oestrus.
Toxoplasma gondii
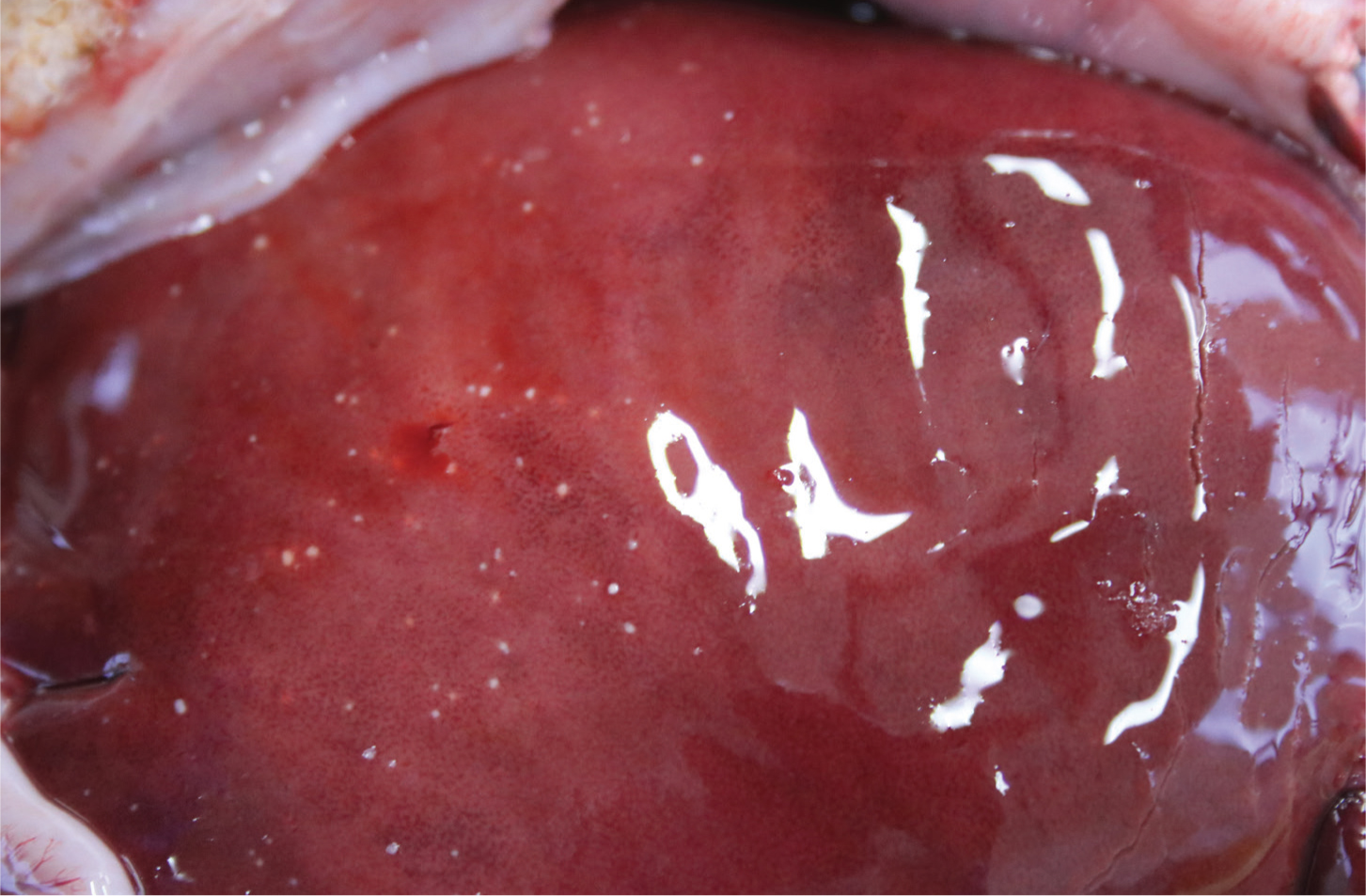
T. gondii is a protozoa that has a complex life-cycle involving a reproductive phase in cats (the definitive host) and a non-reproductive phase in small mammals, birds or potentially sheep (the intermediate host). Young, lactating or immuno-suppressed cats can shed millions of oocytes in their faeces, the infective dose for sheep may only be tens of oocytes so the risk of widespread infection through the ingestion of oocytes from feed, water or the environment is high. Furthermore, oocysts can persist in the environment for up to 12 months. Following infection, tachyzoites invade the placenta causing placentitis and potentially resorption, mummification or abortion (Figure 4) depending on the stage of gestation and infective dose (Benavides et al, 2017; OIE, 2018b). The aborted placenta typically has dark cotyledons with white foci of necrosis. Internally the cotyledons may have a gritty texture if they have mineralised. Between the cotyledons the placenta will appear normal.

Campylobacter fetus fetus/Campylobacter jejuni
Campylobacter is a Gram-negative bacterium that is thought to be spread by carrier sheep, but vector transmission may also be possible. Both Campylobacter fetus fetus and Campylobacter jejuni have been associated with ovine abortion. If infection enters a naïve flock then up to 25% of ewes may abort (Menzies, 2011). If infection occurs during late pregnancy, abortion may occur 7–25 days after infection. Some ewes may develop diarrhoea before aborting. Aborted material is heavily contaminated with infective bacteria and infection can spread rapidly through a flock. Following infection, immunity is long-lived but waves of Campylobacter infection have been observed every 4–5 years, which is thought to coincide with troughs in the levels of immunity in the flock through the birth/acquisition of naïve animals. The gross appearance of aborted material is normal, fetal livers may have grey foci of necrosis. Tetracycline resistance is common among C. jejuni isolates from the USA (Sahin et al, 2008; Wu et al, 2014); to date resistant strains have not been identified in the UK (McGoldrick et al, 2013).
Salmonellosis
Infection with a number of Salmonella sero-types including S. Dublin, S. Montevideo, S. typhimurium and S. abortusovis may result in ovine abortion. S. abortusovis is not currently seen in the UK. Infection is thought to occur via an oral route with birds often blamed for mechanical transmission between flocks (Coulson et al, 1983; Sharp et al, 1983). Carrier animals may transmit S. typhimurium or S. abortusovis. Aborted material and faeces may be heavily contaminated with Salmonella spp., facilitating transmission within a flock or to other flocks via watercourses or vectors. Infection may be asymptomatic. Under the Salmonella zoonosis order of 1989, Salmonella is a reportable disease and cases should be reported to the APHA. If strains that threaten human health are identified, further on-farm investigations may be performed to advise on biosecurity, disease control and prevention of zoonotic transmission (https://www.gov.uk/government/publications/salmonella-in-live-stock-production-in-great-britain).
The clinical signs of salmonellosis will vary with strain:
- S. Dublin and S. typhimurium result in systemic illness with signs of enteric disease. Pregnant ewes may develop anorexia, diarrhoea, septicaemia or death in addition to abortion. Large numbers of lambs may die in the first week of life. Other species including people are at high risk of infection.
- S. Montevideo results in abortion without other clinical signs of illness in the ewe.
- S. abortusovis is not currently seen in the UK but in countries where it is endemic, can be associated with very high rates (up to 60%) of abortion when it is introduced to a naïve flock.
Aborted material has a normal gross appearance. Examination of ewes post mortem may reveal metritis and retained fetal membranes. Dead lambs typically have evidence of abomasitis and enteritis.
Listeria monocytogenes and Listeria ivanovii
Abortion associated with Listeria spp. (L. monocytogenes and L. ivanovii) is not usually accompanied by other clinical signs, but enteritis, septicaemia and neurological listeriosis may be seen. Pregnant animals that are infected may abort as soon as 7 days after infection. Listeria is ingested from decaying vegetation and soil and there is an association with feeding poorly fermented silage and cold wet weather linked to an increase in silage intake or muddy pasture (Low and Donachie, 1997; Mearns, 2007b). Infection may also be introduced by carrier sheep. Aborted material is typically autolysed and the aborted fetus may have miliary yellow/white foci on the liver, circular erosions of the of the abomasum and enlargement of the mesenteric lymph nodes (Figure 5).
Schmallenberg virus
Schmallenberg is a teratogenic orthobunyavirus that was first identified in the UK in 2011–2012 and reappeared in 2016. Infections tend to occur in ‘waves’ every 3–5 years with waning flock immunity and increasing numbers of naïve replacements. Climatic conditions also influence the prevalence of Culicoides midges, which act as vectors for the virus. Infection is generally asymptomatic; however, if it occurs in naïve animals during pregnancy lambs may be stillborn and have abnormalities including arthrogryposis, spinal defects and hydranencephaly. Dystocia is common as a result of the fetal deformities. Infection in early gestation may result in lower conception rates and abortion. As the signs of Schmallenberg virus (SBV) are characteristic, diagnosis is often made on farm and it is well recognised that disease associated with the virus is underreported. In 2016, evidence of SBV was identified on 138 of the 291 farms tested (Stokes et al, 2018). The APHA has recently provided free polymerase chain reaction (PCR) testing for cases fitting the description of SBV.
Coxiella burnetii (Q fever)
C. burnetii is an intracellular rickettsial organism that is now reportable in the UK as a result of its potential to cause severe illness in people, which was demonstrated by a recent outbreak in the Netherlands. While Q fever is considered endemic in the UK, reports of clinical disease in sheep are rare. In a survey of 5791 sheep from 384 flocks performed in 2008, 53 sheep from 37 flocks were found to be seropositive (Lambton et al, 2016).
The spores of C. burnetii survive in the environment and in ticks (which act as a vector) for long periods. Following oral ingestion, the rickettsial organisms localise in the placenta and mammary gland. If abortion occurs it is typically in the last week of pregnancy; infection may also result in stillborn or weak live lambs. Aborted material is contagious. The aborted placenta is covered in exudate and has thickened intercotyledonary areas, i.e. the same as with Chlamydia abortus. The APHA screen all placental samples for C. burnetti from suspect EAE cases and perform serological screening tests to monitor for Q fever. The infective dose for humans is low and may occur through indirect or windborne transmission, as well as through direct contact with lambing ewes. People can develop flu-like symptoms, chronic hepatitis, endocarditis, osteomyelitis and lymphadenitis, and may also suffer from post-viral fatigue.
Border disease
Border disease virus (BDV) is a pestivirus that has increased in prevalence over the past decade and became the subject of routine screening of fetuses by the APHA from December 2019. Since 2002 there have been 596 diagnoses from 19 109 abortion investigations. BDV is most likely to enter a flock through the purchase of persistently infected (PI) animals, with stillbirths and abortions then being evident at the next lambing. The consequence of infection is dependent on that stage of gestation:
- Non-pregnant animals are unlikely to show clinical signs and will develop protracted immunity
- If the pregnancy is <60 days, infection is likely to result in fetal resorption, mummification, abortion or stillbirth. Some fetuses will survive as PIs
- If the pregnancy is 60–85 days of gestation, the outcome is variable. Immune-mediated neurological damage may result in persistent neurological signs. Lambs are likely to have a strong antibody response
- If the pregnancy is >85 days of gestation, the majority of fetuses will survive and will have a strong antibody response. A proportion of fetuses will be aborted or stillborn.
PI lambs that are born to infected ewes will remain positive and shed infection throughout their lives. Some so-called ‘hairy shakers’ have obvious changes in their wool and have tremors and conformational abnormalities such as doming of the cranium and distortion of the limbs. However, some PIs may simply have a slower growth rate and if PI females are allowed to breed they will either abort or produce PI offspring. Following abortion or production of PIs, some ewes may test antibody negative as a result of persistent viraemia. Serological testing of a proportion of the flock (10%) should indicate the presence of disease; in addition, serological testing of youngstock would distinguish the presence of PI and active infection from historical titres in older ewes. Bovine viral diarrhoea virus can also cause border disease in sheep, and BDV can infect cattle.
Neospora caninum
Neospora caninum is a protozoan with a similar life-cycle to T. gondii but is considered an infrequent cause of ovine abortion in the UK. Dogs are the primary definitive host, cattle are the major intermediate host but other species including sheep may also be infected. In 2002, serology was performed on 660 ewes that had aborted and less than 0.5% were seropositive to N. caninum (Helmick et al, 2002). In 2010, fetal fluid samples from 117 aborted lambs were screened for N. caninum with immunofluorescent antibody testing, and a single positive was identified, no positives were identified on immunohistochemistry (APHA unpublished data). In 2015, infection was confirmed in a single aborted lamb that was submitted to the APHA as a suspected SBV abortion.
Leptospira species
Leptospires are Gram-negative bacteria that are considered a rare cause of abortion and agalactia in the UK, but are found more commonly in Northern Ireland. Leptospira hardjo is the serovar that is identified most frequently and 6% of adult sheep were found to be seropositive in 1984 (APHA Zoonosis report (now archived)). The kidneys of 77 aborted lambs were screened for leptospires by PCR in 2011 (APHA unpublished data) and no positives were identified. In the majority of cases, infection is probably subclinical.
Other sporadic causes of abortion
Other infectious agents including Bacillus licheniformis, Trueperella pyogenes, Yersinia, shiga-toxin producing Escherichia coli, mycotic infections and Anaplasma phagocytophilum (tick-borne fever) may also cause sporadic cases of abortion but are unlikely to cause significant economic losses.
Diagnosis of ovine abortion
Most flocks will experience some cases of abortion each lambing so there may be reluctance to investigate every case. However, the consequences of infectious abortion outbreaks can be catastrophic so in the event of multiple cases of abortion a detailed history should be gathered to include information on the flock and specific information on each case of abortion. A definitive diagnosis of abortion can rarely be made from the gross appearance of the fetus or placenta. Ideally, material from every abortion should be submitted to the APHA (or equivalent) for investigation — investigation of each abortion is beneficial, for example, if EAE is identified in a naive flock, preventative measures can be undertaken before the next breeding period — but this may not be feasible because of time and expense. On-farm record keeping is essential so that rates of abortion and the details of each event can be retrieved and analysed (Table 1). Where rates of abortion exceed 2% it is likely that there is an infectious aetiology and investigation is warranted (Mearns, 2007a). Samples submitted for investigation tend to come from smaller flocks which may highlight poor record keeping, greater time pressure or lower perceived value in investigation among those working with larger commercial flocks. Farmers are likely to underestimate the number of lamb deaths if they are not recording them.
Table 1. Information that should be collected when investigating multiple cases of abortion. Modified from Mearns, 2007
| Flock level information | Ewe level information |
|---|---|
| Size and nature of flock | Number of abortions |
| Open/closed flock. Source/number of replacements | Dates of abortions |
| Expected lambing dates | Age/group of the aborted ewes |
| Duration of housing prior to lambing (if applicable) | Are aborting ewes bought in replacements? Which year? |
| Incidence of abortion in previous years, and what/whether diagnoses were made | Are aborted ewes unwell |
| Use of vaccinations for abortion — what and when? | Are aborted lambs of normal size/appearance |
| Pregnancy scanning results (if available) | Were aborted ewes promptly isolated in this and previous years |
| Dates and details of recent stressful events | How was aborted material disposed of |
| Source and storage of feed and bedding | |
| Presence of rodents/cats/cattle |
Aborted material should be double bagged, labelled and placed in a sealed container for transport. Where the whole fetus and placenta cannot be transported, standard samples should be obtained (Table 2) and posted in accordance with postal regulations such that rigid containers are secure within another layer of sealed packaging and are surrounded by sufficient absorbent material. The package must be labelled as a pathological sample with both the sender and recipient's address. Many veterinary practices will process these samples for farmers and forward them for investigation. Where farms are more than 1 hour from an investigation centre, carcass collection will be undertaken free of charge by the APHA. The tests that the APHA (or equivalent) perform on the fetus and placenta in the investigation of ovine abortion are shown in Table 3. Maternal serology may also be performed to determine evidence of exposure and the tests available through the APHA are shown in Table 4. Where a diagnosis is not obtained further, more specialist testing may be performed (Table 5) (Figure 6). Note in Scotland border disease is not tested for unless specifically requested. Further information on the collection and submission of samples to the APHA is available at: http://apha.defra.gov.uk/documents/surveillance/sub-handbook.pdf.
Table 2. Standard samples that should be collected for the investigation of abortion where the whole fetus and placenta cannot be submitted
| Placenta — the section should include at least one cotyledon and have large margins of intercotyledonary membranes |
| Pleural or peritoneal fluid from the fetus |
| Fetal stomach contents collected aseptically using a plain vacutainer and needle |
| Spleen |
Table 3. Diagnostic tests that can be performed by the APHA on the aborted fetus or placenta in the investigation of ovine abortion
| Sample | Test | Cause of abortion tested for |
|---|---|---|
| Placenta including multiple cotyledons and intercotyledonary membrane | Gross examination for placentitis and stained smear | Chlamydia (EAE), Coxiella (Q'fever) and Brucella spp. |
| Polymerase chain reaction (PCR) | Toxoplasma gondii, Chlamydia, Coxiella | |
| Fetal stomach contents collected aseptically via a syringe and needle or with a vacutainer | Bacteriology | Bacterial causes including Campylobacter and Salmonella spp. |
| Fetal fluid from thoracic or abdominal cavity | Antibody iFat | Toxoplasma gondii |
| Spleen/thymus fresh | PCR | Border disease |
| Liver fresh | Additional bacteria | Bacterial causes including Campylobacter |
| Brain fresh | PCR | Schmallenberg virus |
Table 4. Maternal serology tests that can be performed by the Animal and Plant Health Agency in the investigation of ovine abortion
| Maternal blood serum (red top) | Ab enzyme-linked immunosorbent assay (ELISA) | Chlamydia abortus |
| Latex agglutination test | Toxoplasma gondii | |
| Ab ELISA, polymerase chain rection | Border disease, Schmallenberg virus |
Table 5. Specialist testing that can be performed by the Animal and Plant Health Agency in the investigation of ovine abortion
| Test | Causative agent | Location |
|---|---|---|
| Maternal serology | Border disease enzyme-linked immunosorbent assay (ELISA)Q fever ELISALeptospira hardjo | MoredunWeybridgeWeybridge |
| PCR | Anaplasma phagocyophilum (TBF) polymerase chain reaction (PCR) (TC0047) can be done on EDTA blood and tissues from spleen, lymph nodes and lungs. Maternal samples are the choice material | Moredun |
| Pathogenic leptospires on fetal kidneyChlamydia spp. PCR on placenta if modified Ziehl–Neelsen stain negativeCoxiella burnetii (Q fever) on placental cotyledon or fetal fluid | Penrith | |
| Schmallenberg virus (TC0905) at least 1 g tissue, brain preferred | Weybridge | |
| Histopathology | Placenta (cotyledonary and intercotyledobary areas), fetal lung, heart, liver, kidney, thyroid and brain | Weybridge |
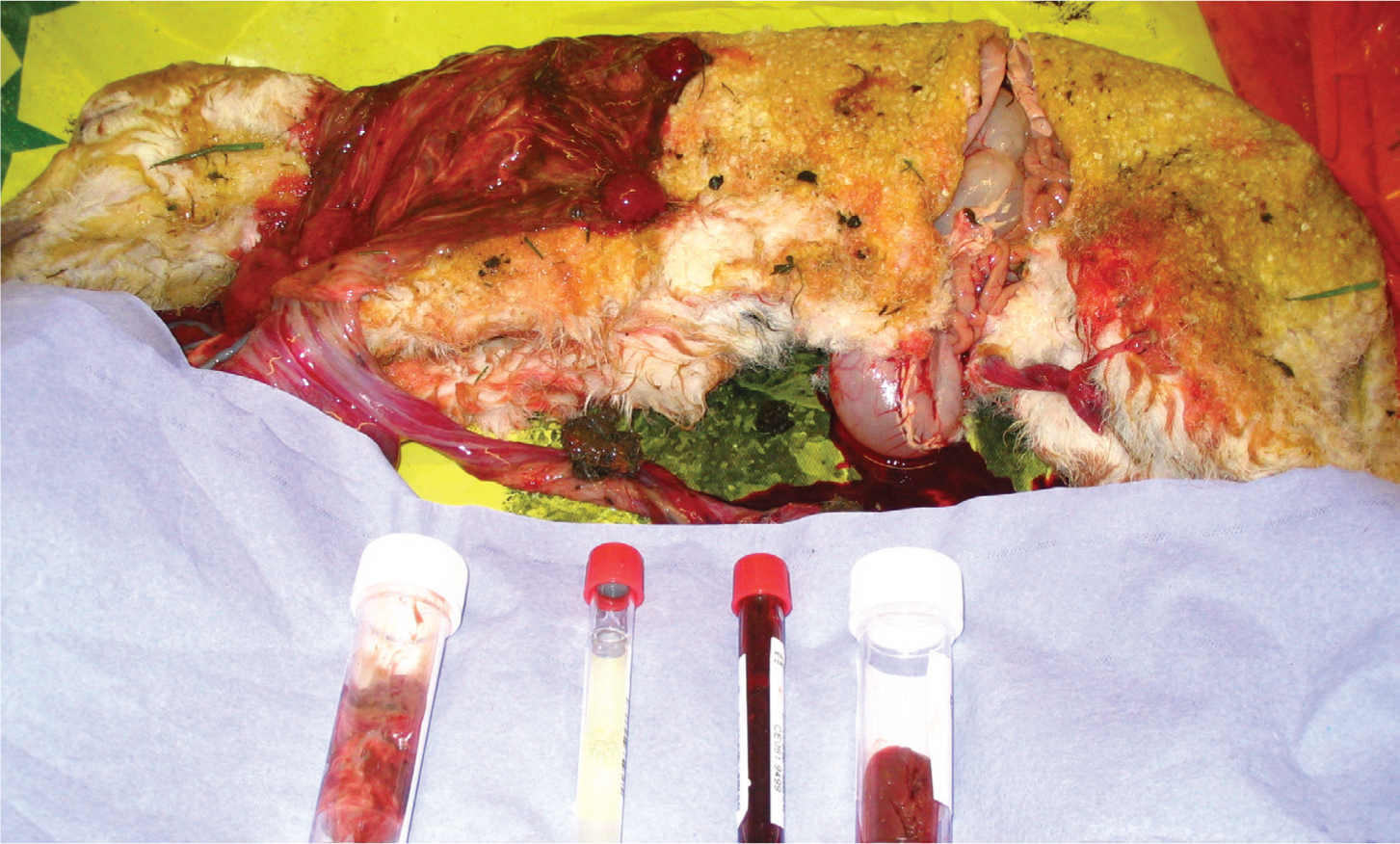
There is a tendency for farmers and veterinarians to focus on the number of abortions within a flock. However, it is important not to overlook flocks that have a high prevalence of small, weak, ill-thrift lambs as a consequence of infectious placentitis. Losses associated with large numbers of weak lambs with poor growth rates may be more insidious, but ultimately more significant than with small numbers of still born or aborted lambs.
The APHA and its partners apply a standard testing protocol to abortion material that is received with the cost being subsidised:
- Fetuses are examined systematically for gross abnormalities
- A smear of placenta is collected and examined for Chlamydia spp. and Coxiella burnetii and results are reported immediately
- Smears of placenta and stomach contents are examined for evidence of Brucella spp. in order to maintain the UK's status as free of this disease
- Examination of stomach contents allows detection of fungal hyphae and may also be stained for C. abortus
- Cultures of fetal stomach contents and fetal tissues are prepared to investigate the presence of Campylobacter spp, Salmonella spp., Trueperella pyogenes, Yersinia spp., Bacillus licheniformis, Listeria spp. including monocytogenes, and E. coli. Histology may be used to confirm the significance of bacteriological findings
- Samples are collected for PCR testing for border disease (not Scotland) and T. gondii
- Fetuses with arthrogryposis are tested for SBV
- If no diagnosis is obtained, additional testing may be considered to include Anaplasma phagocytophilum (tick-borne fever) and Leptospira spp.
If a diagnosis remains elusive then antibody screening of ewes may be performed (Table 4) and on-farm visits can be undertaken by APHA veterinary investigation officers. Details of how to contact the APHA are shown in Table 6.
Table 6. Contacting the APHA
|
KEY POINTS
- There are many causes of ovine abortion in the UK.
- Chlamydia abortus (enzootic abortion in ewes (EAE)) is the most frequent diagnosis of ovine abortion.
- Abortion due to EAE typically occurs in the last 3 weeks of gestation.
- Aborted material as a result of EAE will be heavily contaminated with the infectious organsim.
- Toxoplasmosis is the second most common cause of ovine abortion.
- Infection with Toxoplasma gondii is through digestion of the parasite oocytes often as a result of ingestion of cat faeces.
Reducing the risk of abortion
Measures to reduce the risk of abortion should be discussed as part of the development of a flock health plan. Discussion should also cover the collection of production data to allow benchmarking; this will identify when an abortion problem is occurring, even if the farmer does not perceive there to be an issue. Each flock needs to be considered in its own right and messaging targeted to the farm's specific circumstances. There are multiple benefits to reducing the prevalence of abortion and different farms and farmers will have different motivators:
- Greater profitability through sales of greater numbers of lambs and reduced costs at lambing
- Greater value for accredited breeding stock
- Reduced labour costs associated with abortion and isolation of ewes and and the nursing of weak or sick lambs
- Improved mental wellbeing and reduced stress through improved flock health and reduced sheep losses
- Enhanced reputation for the farm and the industry
- Reduced antimicrobial useage
- Reduced risk of illness for those working with the flock.
There are challenges in connecting with sheep farmers who elect to have minimal veterinary involvement on their farms, and wider government and industry efforts are required to improve their engagement. Proposed interventions should take into account the motivators of human behaviour and be SMART — specific, measurable, achievable, realistic and timely. Effective communication between veterinary surgeon and farmer is critical to success. If farmers can engage with the benefits and be empowered with understanding of the changes that are required to reduce the prevalence of abortion then there is a greater chance of success. The interventions that are typically required to protect a flock are relatively straightforward and inexpensive; however, the barriers to implementing them need to be identified and broken down for change to result. To understand the barriers it is generally better to get onto the farm and discuss the misgivings and specific practical challenges that are perceived. A range of risk reduction measures should be discussed:
- Running a closed flock. Definitions of ‘closed’ can vary and it is important to establish exactly what sheep are being brought onto the farm.
- For many farms, running a closed flock is impractical and farmers should then be encouraged to obtain replacements from sources that have higher health status, preferably farms that are accredited free of EAE. Buying off farm reduces the risk compared with buying through market and reducing the number of sources further reduces the risk of bringing in disease.
- Running and lambing replacements away from the main flock for at least one lambing and preferably keeping them in the groups in which they were sourced until after they have lambed.
- Not introducing orphan lambs to the flock that can serve as a source of EAE.
- Vaccination should be discussed and protocols agreed that take into account the level of risk to the flock.
- Prompt isolation and recording of any ewes that abort, and appropriate disinfection of the environment and disposal of aborted materials. Specific instruction on effective disinfection should be discussed and protocols agreed with all relevant staff. Appropriate areas should be identified in which all aborted ewes can be isolated until discharges dry up (Table 7).
- Investigation of the cause of all (preferably) cases of abortion or agreement of protocols on when investigation should be performed. Investigation is generally recommended if more than 1–2% of the flock abort but if abortions are not investigated as they occur the overall percentage may not be known until it is too late.
Table 7. Steps to take following abortion
| 1. | Isolate the aborted ewe for at least 3 weeks or until all discharges have dried up |
| 2. | Dispose of aborted material and contaminated bedding or feed |
| 3. | Disinfect the pen and/or immediate area |
| 4. | Assess the health of the ewe and consider antimicrobials if fetal membranes are retained or there are signs of systemic infection such as pyrexia |
| 5. | Do not foster lambs onto aborted ewes, particularly ewe lambs that might be considered as future breeding animals |
| 6. | Mark/record the identity of aborted ewes |
| 7. | Submit aborted material for investigation including placenta |
| 8. | Blood sample all barren and aborted ewes at the end of the lambing period |
| 9. | Review and potentially revise biosecurity protocols for future abortions |
| 10. | Do not allow pregnant women near the lambing shed |
KEY POINTS
- Flock health plans should include advice on reducing the risk of abortion.
- Benefits of reducing the risk of ovine abortion include greater profitability, reduced labour costs, reduced zoonotic risk and improved farmer mental wellbeing.
- Reducing the risk of ovine abortion can help reduce dependence on antimicrobials.
- Effective communication is critical to success.
- Vaccination against enzootic abortion in ewes (EAE) is like investing in insurance that means that an EAE abortion disaster will not happen.
Vaccination
As part of the delivery of RUMA (responsible use of medicine in agriculture) targets, the English levy board (the Agriculture and Horticulture Development Board (AHDB)) collate vaccine sales data and AHDB estimate rates of ovine abortion vaccination from vaccine sales data (Figure 7). The imperfect denominator is the number of ewes that farmers report they are retaining for first time breeding in the annual Department for Environment, Food and Rural Affairs (Defra) census. The total number of replacements is typically reported to be around 2.5 million, which is considered to be low given other estimates of there being 15 million ewes in the UK, with most commercial flocks replacing 25% of their ewes each year. Regrettably there is no information on the proportion of open versus closed flocks or those that lamb indoors versus outdoors. Despite the limitations of the data, the methods are consistent year on year and relative differences in rates of vaccine uptake should be evident. The data consistently indicate that levels of vaccination for EAE and Toxoplasma spp. are low, peaking in 2020 at 50% and 31% respectively. Options for vaccination are summarised in Table 8.
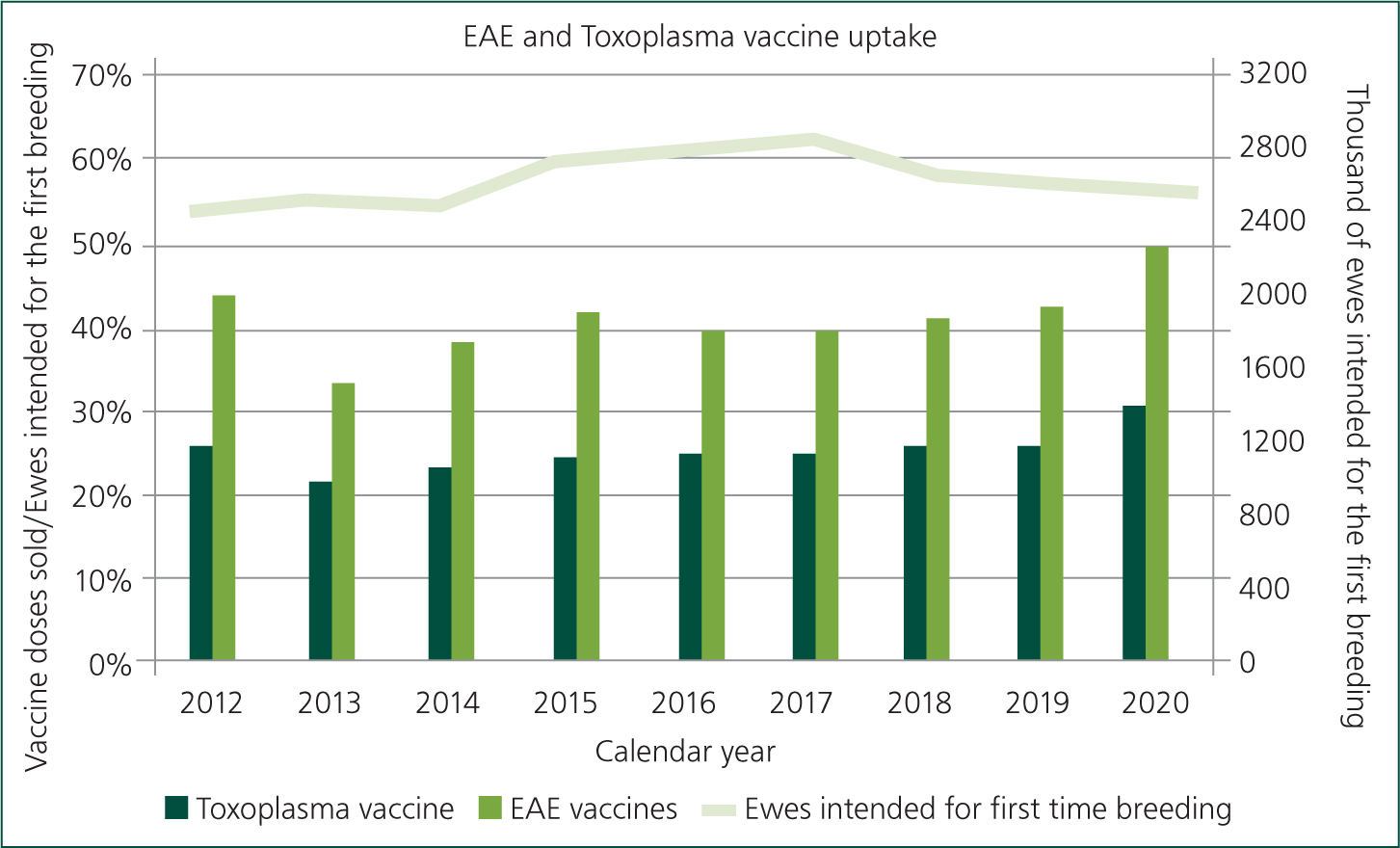
Table 8. Vaccines that are available in the UK for protection against abortion. Modified from Mearns, 2007.
| Disease | Vaccine | Type | Dosage | Storage | Duration of protection | Indications | Contraindications | Meat withdrawal | Precautions |
|---|---|---|---|---|---|---|---|---|---|
| Enzootic abortion in ewes (EAE) | Enzovax (MSD) | Live attenuated | 2 ml IM or SC | 2-8°C out of direct sunlight. Use within 2 hours of reconstitution | At least 3 years | Replacements >5 months of age. It is recommended animals are vaccinated in the period between 4 months and 1 month before tupping. May be administered with Toxovax at different sites | Not for use during pregnancy or within 4 weeks of tupping. Do not use in association with antimicrobials, especially tetracyclines | 7 days | Avoid self-injection. Not to be used by women of childbearing age or immunocompromised individuals. Wear gloves |
| Cevac Chlamydia (Ceva Animal Health Ltd) | Live attenuated | 2 ml IM or SC | 2–8°C out of direct sunlight. Use within 2 hours of reconstitution | Not stated | Replacements >5 months of age. It is recommended animals are vaccinated in the period between 4 months and 1 month prior to tupping. May be administered with Toxovax at different sites | Not for use during pregnancy or within 4 weeks of tupping. Do not use in association with antimicrobials. Do not use within 4 weeks of other live vaccine | 7 days | Avoid self-injection. Not to be used by women of childbearing age or immunocompromised individuals. Gloves recommended | |
| Toxoplasma | Toxovax (MSD Animal Health) | Live tachyzoites | 2 ml IM | 2–8°C. Use within 2 hours of reconstitution. Note short expiry date. | At least 2 seasons. With additional natural exposure potentially lifelong | Replacements >5 months of age. should be vaccinated in the period from 4 months to 3 weeks prior to tupping | Not for use during pregnancy or within 3 weeks of tupping. Can be given at the same time as live attenuated EAE vaccines at different sites | 42 days | Gloves and eye protection should be worn during reconstitution. Risk of zoonotic infection |
| EAE and Salmonella abortusovis | Inmeva (Hipra) | Inactivated | 2 ml SC | 2–8°C out of direct sunlight. Once opened, use within 10 hours | 1 year-An annual booster at least 2 weeks before each subsequent mating is required | Two doses, 3 weeks apart, the first at least 5 weeks before tupping. Can be used during pregnancy. Not recommended in the last month of pregnancy | Use in last month of gestation not recommended | Nil | Avoid self-injection |
| Campylobacter fetus fetus and Campylobacter jejuni | Campyvax 4 (MSD, not registered in the UK, but can be imported under licence from the VMD) | Inactivated | 1 ml SC | 2–8°C out of direct sunlight. | 12 months | Two doses 4–8 weeks apart at least 4 weeks before first mating followed by annual boosters. | Statutory meat withdrawal period (has 0 day withdrawal period in country of origin) | Avoid self-injection |
Work performed by MSD in 2014 indicated that 81% of flocks had been exposed to T. gondii 52% to EAE and 43% to both organisms. Exposure to T. gondii has been more robustly demonstrated; in 2009 a study of 3539 ewes indicated that seroprevalence increased from 20% to 64% between 1 and 2 years of age and peaked at over 80% (Hutchinson et al, 2011). Considering this high level of risk and the costs associated with abortion, there is a strong economic argument that all flocks should be vaccinating for T. gondii in addition to there being clear benefits for ovine and human health. Given the potential for widespread losses as a result of EAE, all flocks that are bringing in replacements should be vaccinating replacements for EAE, including those that are sourcing replacements from EAE accredited flocks. In closed flocks that do not have other flocks as direct neighbours, it is reasonable to be diligent with biosecurity and to be proactive in investigating all cases of abortion rather than vaccinating for EAE as a matter of routine. Investigation of UK flocks that had had EAE over 4 or more years demonstrated that failure to vaccinate was a consistent feature (Carson et al, 2019).
It is common practice to vaccinate ewes only once in their lifetime, before first tupping, although the vaccine data sheets have either no specific claim for duration of immunity (Cevac Chlamydia, CEVA) or 3 years' duration of immunity (Enzovax, MSD). As the cost of the vaccine is thus spread over the entire lifetime of the ewe, the expense considered on a per year basis is relatively small. Against a cost of around £3–4 per ewe for EAE vaccination, the cost of endemic EAE can easily be £25 per ewe (Silk, 2016) in the flock, removing any profit from the farming enterprise. Estimates of the cost of each aborted ewe ranged from £122 to £177 in one study (Robertson et al, 2018). Anecdotally, there is a tendency for farmers to overlook the hidden costs of abortion and to focus on the fixed cost of vaccination, but with 80% of the farm's investment in its ewes having been made by lambing time, the cost of vaccination is small and helps to maximise return on investment. Furthermore, the cost difference between vaccinating for both EAE and T. gondii and vaccinating for T. gondii alone is small, making protection against EAE even more economical. There is an increasing trend toward selling ewe lambs that have been vaccinated and these animals may command a premium; however, purchasers need to be confident that the seller has handled and administered the vaccine appropriately.
Veterinary surgeons should discuss the merits of vaccination for abortion and incorporate protocols into flock health plans. Furthermore, practices should be proactive in reviewing practice management systems and contacting farmers a few months before tupping to discuss the benefits of vaccination and to remind them to schedule it into their plans. This is especially pertinent if they are known to have had cases of abortion during the preceding lambing. Few (if any) practices send vaccination reminders for sheep flocks despite sending regular reminders for the single sheep dog!
The use of bovine viral diarrhoea (BVD) vaccine for the prevention of border disease is controversial and the evidence for conferred immunity is mixed. The varied results may reflect different responses to different strains of virus. BVD has been reported as a cause of congenital deformity and abortion (Hopkins et al, 2019) and studies performed in the 1980s and 90s indicated that up to 20% of border disease cases may in fact be becuase of BVD and in these cases the BVD vaccine may be effective. Anecdotally in Ireland the prevalence of border disease has reduced with greater use of BVD control measures.
KEY POINTS
- All flocks that bring in replacement ewes or have neighbours that lamb sheep, should vaccinate to protect against enzootic abortion in ewes (EAE).
- Sourcing replacements from EAE accredited flocks reduces the risk of bringing in EAE.
- Bought in ewes should be lambed separately from the home flock for their first year to contain issues (especially EAE or border disease).
- Exposure to Toxoplasma gondii is widespread, and so flocks are likely to benefit from vaccination with Toxovax.
- Veterinary surgeons should discuss the merits of vaccination for abortion and incorporate protocols into flock health plans.
- Practices should be proactive in contacting farmers a few months before tupping to discuss the benefits of vaccination.
Use of antimicrobials
The benefits of antibiotic treatment in the face of abortion are dependent on the cause. Only in the case of EAE is there any evidence to support the metaphylactic use of antiobiotics. The responsible use of medicines in agriculture (RUMA) targets task force of 2017 indicated that antimicrobials were used routinely to control abortion in 10% of sheep flocks (Phillips et al, unpublished data). This approach is neither ethical nor sustainable. The ‘Plan, Prevent, Protect’ initiative was launched by the sheep antibiotic guardian group to reduce reliance on antimicrobials in the management of EAE (https://www.farmantibiotics.org/tool_links/target-enzootic-abortion-in-ewes-this-year-and-cut-your-losses/Figure 8). The use of antimicrobials for the prevention of abortion is specifically addressed in the RUMA industry guidance document for veterinary surgeons and farmers on responsible use of antibiotics in sheep (https://www.ruma.org.uk/wp-content/uploads/2019/10/RUMA-Sheep-Antibiotic-Use-Good-Practice-Guide-July-2019.pdf).
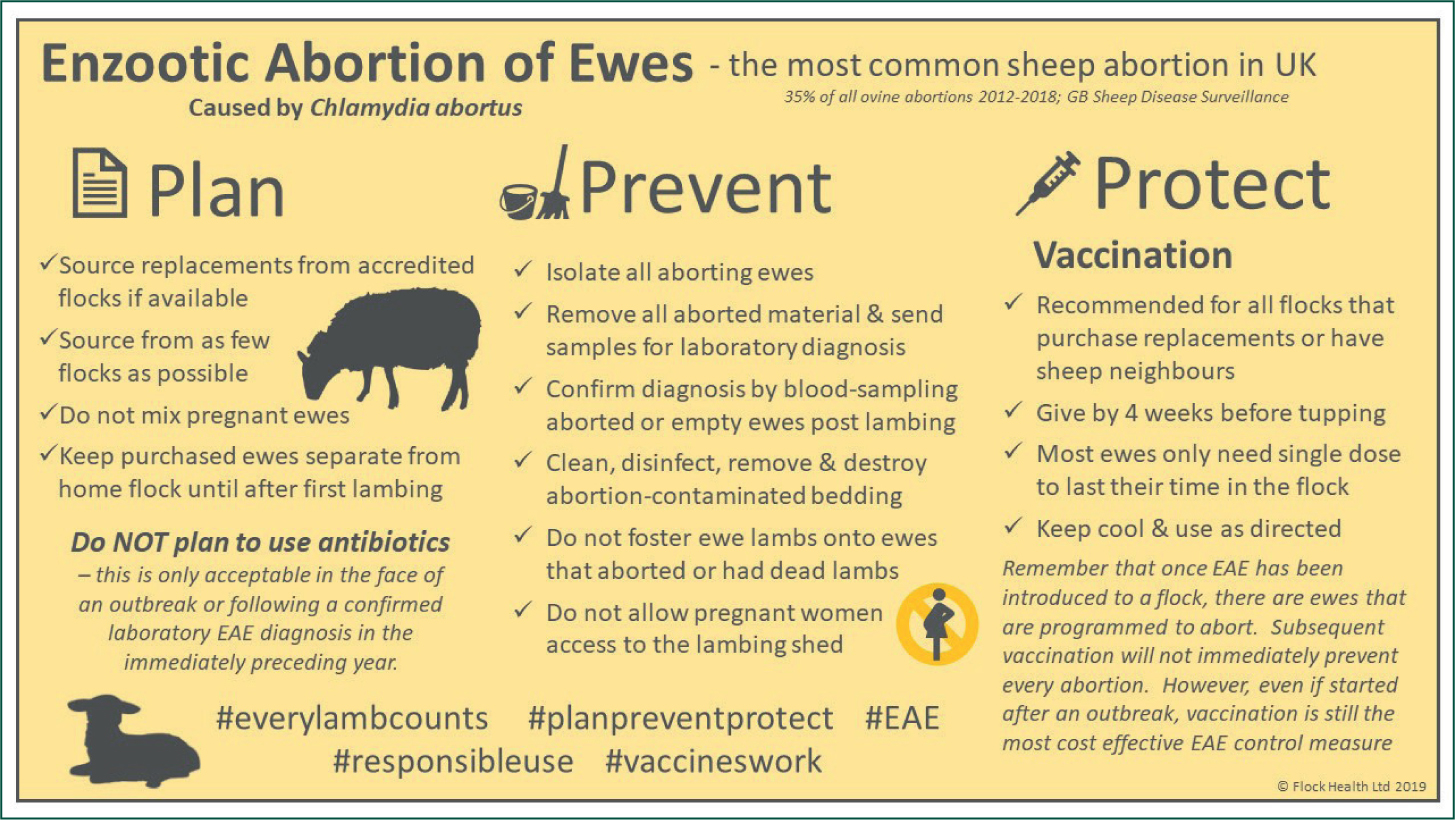
It is not acceptable to use routine antibiotic treatment in the period of late pregnancy as a control measure for abortion in general — i.e. in any flock unless in the face of an outbreak of EAE or if there has been a confirmed laboratory diagnosis of Chlamydia abortus in the immediately preceding year.
In the immediate face of a new outbreak, if it is not possible to use an inactivated vaccine, it is acceptable to treat the affected group of ewes with injectable long-acting oxytetracycline. It is also acceptable to use this antibiotic treatment for later lambing ewes within the flock, when they reach the period between day 90 and day 126 of that pregnancy or at the same stage for the affected group of ewes during their following pregnancy.
When antimicrobials are used to prevent EAE abortion, oxytetracycline is typically used at 20 mg/kg. It is important to consider the increased in weight of heavily pregnant ewes in order to prevent underdosing (Figure 9).
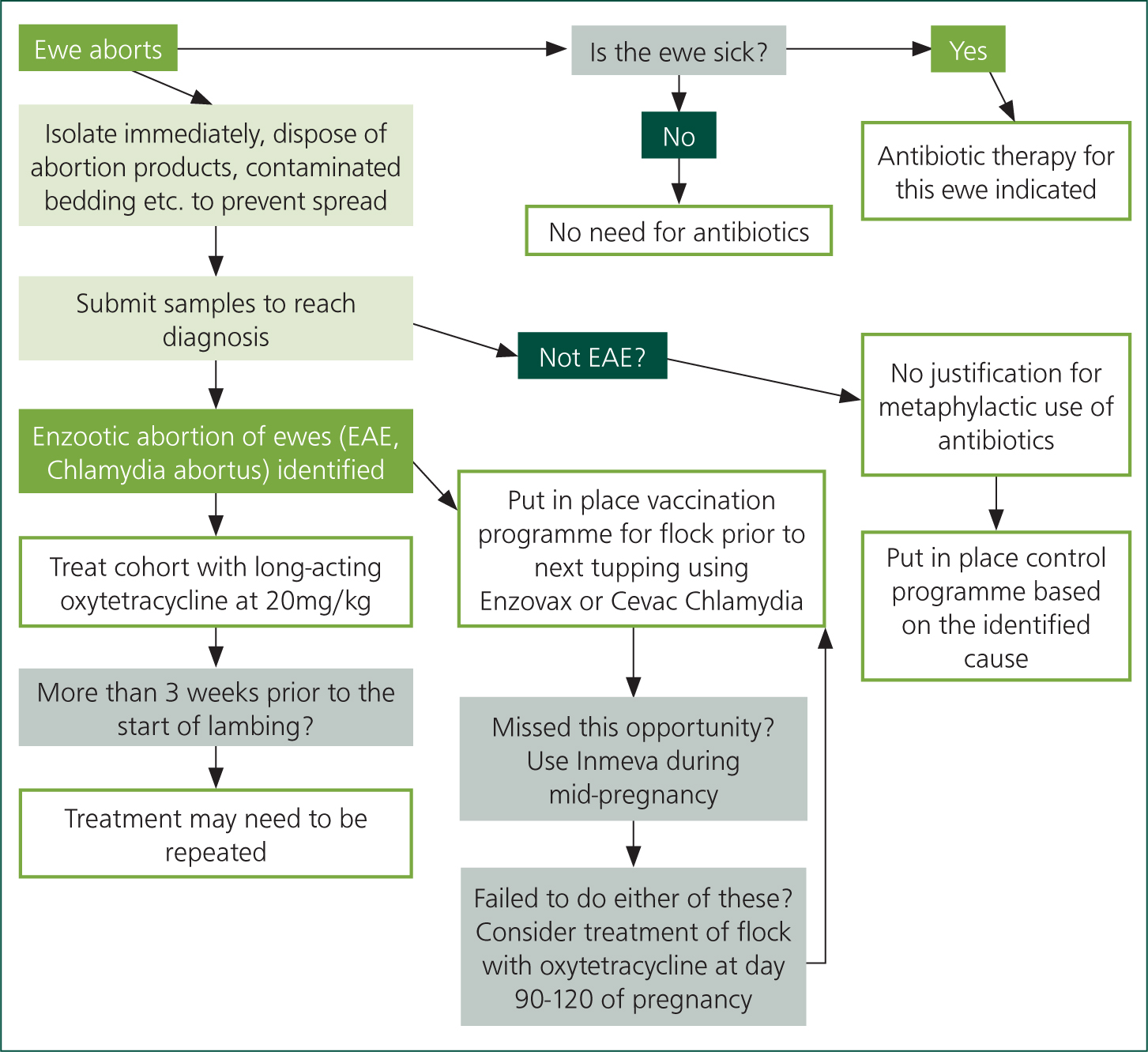
It is not acceptable to use antibiotics to control abortion on an ongoing basis. Antibiotic treatment of ewes in late pregnancy, generally using a long-acting oxytetracycline, may help to reduce the number of ewes that abort but it does not reduce the shedding of Chlamydia abortus or reduce the incidence of infected ewes within a flock. Neither is this a cost-effective approach when compared with vaccination over the medium to long term.
Whole flock, prophylactic antibiotics are not considered necessary nor appropriate for control of EAE in sheep flocks.
Conclusions
The tools are available to dramatically reduce the prevalence of ovine abortion and there are clear economic and ethical arguments for using them. The challenge comes in persuading farmers that the visible costs associated with implementing better biosecurity and vaccination programmes are far less than the invisible costs of lost lambs and lower lamb growth rates and barren ewes. Effective communication between veterinary surgeons and farmer are essential as is an understanding of human behaviour and the different factors that motivate different farmers.
KEY POINTS
- Following a diagnosis of enzootic abortion in ewes, antimicrobials can be used to prevent further abortions in the flock.
- The blanket use of antimicrobials contravenes the principles of antimicrobial stewardship.
- The use of antimicrobials for the prevention of abortion in sheep is specifically addressed in the RUMA industry guidance document for veterinary surgeons (https://www.ruma.org.uk/wp-content/uploads/2019/10/RUMA-Sheep-Antibiotic-Use-Good-Practice-Guide-July-2019.pdf).

