The selective treatment of clinical mastitis is based on the now widely accepted evidence that the self-cure rate of many Gram-negative bacteria is high without the need for antibiotic therapy (Mansion-de Vries et al, 2016; Lago and Godden, 2018). This allows for more targeted treatment of Gram-positive bacteria, while it may be appropriate for Gram-negative bacteria to be treated without antibiotics. The recent advances and increasing availability of cow-side tests to differentiate between a Gram-positive and Gram-negative bacteria mean that this decision-making process can be based on a test result in conjunction with a wider risk assessment from a mastitis pattern analysis.
Potential benefits of selective treatment of clinical mastitis
The principal benefit of carrying out selective treatment of clinical mastitis is in supporting the One Health approach to the responsible use of antibiotics through the reduction of antibiotic use and targeted antibiotic therapy. Pathogens that do not need antibiotic therapy to increase the likelihood of cure, primarily those with high self-cure rates such as some Gram-negative pathogens, do not receive antibiotic treatment. This will reduce the overall use of antibiotics on a unit, particularly where there is a high proportion of mastitis cases caused by Gram-negative bacteria. Cases that have Gram-positive bacteria identified can be treated with a targeted narrow spectrum antibiotic as part of the treatment plan. The narrow spectrum antibiotics for mastitis intramammary treatment all fall within the European Medicines Agency classification D (Table 1), for prudent use as first-line therapy wherever possible.
Table 1. Lactating cow intramammary products currently available by European Medicines Agency (2023) category
| Brand name | Ingredients | Spectrum of activity |
|---|---|---|
| European Medicines Agency category D | ||
| Orbenin LA (Zoetis) | 200 mg cloxacillin | Narrow – Gram positive |
| Procapen (Forte) | 3.0 g procaine penicillin | |
| Ubropen (Boehringer Ingelheim) | 600 mg benzyl penicillin | |
| European Medicines Agency category C | ||
| Albiotic (Huvepharma) | 330 mg lincomycin100 mg neomycin | Broad – Gram positive and Gram negative activity |
| Mastiplan LC (MSD) | 300 mg cefapirin sodium20 mg prednisolone | |
| Synulox LC (Zoetis) | 200 mg amoxicillin trihydrate50 mg clavulanic acid10 mg prednisolone | |
| Ubrolexin (Boehringer Ingelheim) | 200 mg cefalexin100 000 IU kanamycin | |
Carrying out testing on farm primarily requires a test that is rapid and accurate. However, the test must also be easy to perform, cost-effective, easily stored and the results straightforward to assess. There are several tests that are currently available in the UK market which meet this criteria. These include MastDecide, Mastatest, Vetscan Mastigram+ and VetoSlide. A full discussion of the tests is beyond the scope of this article, therefore readers are directed towards the manufacturers of the tests for full information or the supplementary material in the article by Malcata et al (2020).
Potential disadvantages of selective treatment of clinical mastitis
There are three main areas of concern around carrying out selective treatment of clinical mastitis on farms.
1. Delayed treatment of clinical cases
The delayed treatment of clinical cases is a significant and justified concern. It is widely acknowledged that prompt, effective treatment maximises the chances of a successful outcome, particularly in new or first cases. One of the main considerations in choosing a detection test for use on farm should be how long the test takes to achieve a result and how this can be incorporated into a treatment regimen. There are two areas of focus for vets to concentrate their efforts in understanding and training as part of this discussion:
- How and when are cases detected? The fundamental principal in any approach to treating clinical mastitis is through prompt and effective therapy, possible only when clinical mastitis cases are detected quickly. Most cases are detected during milking, either by milking staff or through sensors or other equipment within the milking parlour. A useful exercise on farm is to gather together all of the people that are involved in the milking to ensure that there is consistency and best practice with how mastitis cases are detected – how milk is assessed and evaluated, what changes are being observed, how the udder is checked and how this information is recorded and communicated so that treatment can be given. It is likely that on many farms the actions following a diagnosis of a case of clinical mastitis can be improved at this stage to reduce the time to the treatment being instigated and improve treatment success.
- When are cases treated? It is common for a case of clinical mastitis to be detected at one milking and then treatment instigated in the next milking – this allows the milking staff time to gather together the required materials for starting treatment, particularly if this is intramammary therapy. Sometimes, a ‘watch and wait’ plan is put in place with cases only being treated if they are symptomatic at the second milking. In either of these two examples, the inclusion of a test at the first point of detection would not delay treatment from the present regimen and in both cases the administration of a non-steroidal anti-inflammatory drug early after diagnosis could be justified and beneficial for clinical mastitis cases.
It is important to remember that all farms are different and many staff behave differently if protocols are not clearly defined. It is a very worthwhile exercise to have these discussions on farm as part of the opportunity that selective treatment of clinical mastitis brings to mastitis control. Incorporating a test that does not disrupt or delay treatment of the mastitis case has obvious advantages and is therefore recommended.
2. Not treating a case which would benefit from antibiotic therapy
This falls into two main categories:
Gram-negative pathogens that are not treated with antibiotics based on the high likelihood of self-cure but do not self-cure
This is a significant consideration – Gram-negative bacteria include Escherichia coli, Klebsiella spp., Serratia spp. and Pseudomonas spp. among many more, and they each have varying characteristics and are capable of different behaviour on farm (Schukken et al, 2012). The most common Gram-negative bacteria isolated from clinical mastitis cases are E. coli, which is an environmental pathogen, generally (but not always) associated with high self-cure rates of mild to moderate cases. Klebsiella spp. infection is more likely to present as more severe cases of longer duration and longer milk production losses post infection, on occasions it has demonstrated contagious transmission on farm (Munoz et al, 2008) and treatment efficacy with antibiotics is often very low. It is possible that Gram-negative bacteria such as E. coli and Klebsiella spp. do behave as persistent pathogens on some farms and this behaviour should be identified from treatment records to determine whether selective treatment of clinical mastitis cases would be an appropriate approach and to monitor cure rates closely if selective treatment of clinical mastitis is instigated. This would present as a low self-cure rate and/or a high recurrence rate, with corresponding bacteriology reports to support the diagnosis. Close monitoring of cure rates, such as the percentage of first cases that do not recur with clinical or subclinical (cell count >200 000 cells/ml) infections within the next 3 months following a case, would help identify individual farms where this is a problem. In all situations, monitoring of the cure rates for both treated and untreated animals is crucial in ensuring the chances of treatment success are maximised.
False negatives
A false negative or no-growth sample can occur with any testing method where the bacteria is present but below detection thresholds. However, no-growth results may also be as a result of non-infectious causation, infection already cleared by the immune defences, errors in sample collection/preservation/storage, reduced viability in the sample or testing method, or, because the organism cannot be detected by the test, eg a virus, Mycoplasma spp. or anaerobic bacteria. Any test should be chosen that minimises the number of false negative results, ie a test with high specificity.
3. Treating a case that does not require antibiotics
This could occur in the event of a false positive result or when the result is a true positive but from a pathogen that may not require antibiotic therapy. The presence of a bacteria is not necessarily indicative of causation of the clinical case of mastitis. This could be as a result of sample contamination or could be from the presence of clinically non-significant bacteria, eg some non-aureus Staphylococci (NAS) (Valckenier, 2020). Sample contamination can be minimised through good sampling technique, and its frequency can be monitored through performing occasional testing that would detect contamination, for example bacterial culture. The presence of bacteria that are non-significant and may not be pathogenic is difficult to assess without full bacterial identification. This can be done before embarking on a selective treatment of clinical mastitis protocol with a farm through routine surveillance or may be detected through occasional bacteriology testing once a protocol has been in place.
Evidence from the literature
Terminology
The term ‘selective’ is already an area of debate, with some favouring the term ‘targeted’ treatment. Within the literature, selective treatment around antimicrobial use is defined as ‘the practice of restricting antimicrobial use to cases most likely to benefit from treatment and may help to reduce antimicrobial use’ (Malcata et al, 2020). This paper goes on to describe that ‘selective treatment’ refers to blanket treatment as the default option, with this approach being modified to select cows for treatment. Targeting, the authors suggested, describes no treatment as the default option, with targeting of treatment to those animals that are most likely to benefit (Malcata et al, 2020). While the term ‘targeted’ may be more favourable to those outside of the industry, notably the general public, we must be careful that our producers do not feel confused or pressurised in to withholding antibiotics without a form of testing to effectively allow or justify their use. This is because the majority of clinical mastitis cases are bacterial in origin, where prompt and effective treatment maximises cure rates to aid resolution, reduce the chances of recurrence and reduce the potential spread of infection. This contrasts with many other common human conditions which are either viral in origin or self-resolve without antibiotics in many cases, and where targeted therapy could therefore be a more appropriate term. The phrase ‘selective treatment of clinical mastitis’ is now widely used in the scientific published literature and is therefore used in this article. Targeted treatment is used alongside the term narrow spectrum to describe antibiotic therapy that does not have activity against a broad range of bacteria.
Published work
There are several published papers assessing or reviewing the differences in cure rates between blanket and selective treatment of clinical mastitis regimes.
There were two published review papers in 2023 on the selective treatment of clinical mastitis. These were both principally written to answer the question as to whether the selective treatment versus the blanket treatment would demonstrate similar udder health outcomes – a first do no harm approach. The systematic review and meta-analysis of selective treatment of clinical mastitis (de Jong et al, 2023a) identified 13 studies that compared blanket treatment and selective treatment of clinical mastitis and found that the selective treatment of clinical mastitis cases was not inferior to the blanket treatment protocol for the outcome bacteriological cure. They also presented a not clinically relevant increase in interval from treatment to clinical cure (of 0.4 days) in the selective group and a higher proportion of clinical cure at 14 days in the selective group. Overall, they concluded that selective treatment of clinical mastitis did not adversely influence bacteriological cure, clinical cure, somatic cell count, milk yield, incidence of recurrence or culling (de Jong et al, 2023a). Selective treatment of clinical mastitis should be guided by the use of rapid and accurate diagnostic tests to aid case selection. None of the studies were based on UK herds.
The invited review on selective treatment of clinical mastitis (de Jong et al, 2023b) described the main purpose of selective therapy as being treatment of only cases with a high probability of being responsive to antimicrobials. Cases where the likelihood of intramammary therapy is low includes cases caused by Staphylococcus aureus or Streptococus uberis that have been preceded by elevated somatic cell count levels, particularly if they have been persistently elevated. The review stated that treatment of severe cases should not be delayed by the use of a diagnostic test, however a test could be used alongside treatment if it may lead to revision of the initial treatment plan when the result is available (de Jong et al, 2023b). The review contains a section of economic consequences of selective decision making, however the treatment scenarios presented are not appropriate to the UK situation because of the licensed products that we have available. The main conclusion relating to the cost benefit that can be applied to our herds is that the return on investment will depend on the proportion of cases that do not have a Gram-positive organism identified, this is because the cost of the test is likely outweighed by not applying antibiotic treatment and having a subsequent milk withdrawal period. It will also depend on the treatment protocol used for Gram-positive cases and the cost of this treatment and appropriate withdrawal period. Thus, herds with a higher proportion of Gram-negative (or no growth) cases are more likely to see a greater return on investment when performing selective treatment regimes. A final relevant conclusion of this invited review was that the co-administration of non-steroidal anti-inflammatory drugs to clinical mastitis cases did improve outcomes (de Jong et al, 2023b).
The most valuable of the published papers comparing outcomes of selective treatment of clinical mastitis in peer-reviewed literature are from those studies that performed randomised clinical trials, and while they are included in the review papers above, they are summarised in this section and Table 2. These papers use a superiority approach, designed to show that one treatment option is better than another, compared with the systematic review and meta-analysis which uses a non-inferiority approach, to show that one treatment is no worse than another. The other papers that are included in the meta-analysis review are not discussed further here because of one of the following reasons:
- They have not been published through a peer-reviewed paper
- They include severe cases in the selective treatment group and/or they have different pathogens between the selective and blanket treatment groups or there is concern regarding bias in the randomisation process
- Similarly, papers where the treatment protocols were not followed between the groups are not reviewed further here.
Table 2. Published papers evaluating blanket versus selective treatment of clinical mastitis cases
| Published paper | No of cases | Percentage of cases receiving antibiotic in STCM group | Bacteriological cure (%) | Days to clinical cure | Test used and treatment protocol | ||
|---|---|---|---|---|---|---|---|
| Blanket | Selective | Blanket | Selective | ||||
| Lago et al (2011a; b) | 449 (8 herds) | 44 | 71 | 60 | 2.7 | 3.2 | Minnesota Easy Culture System II Bi-plate (18–24 hours incubation). Treatment all groups - 2 tubes intramammary cephapirin sodium, 12 hours apart |
| Vasquez et al (2017) | 489 (1 herd) | 32 | N/A | N/A | 4.8 | 4.5 | Standard laboratory culture technique under NMC guidelines (18 – 24 hour incubation). Treatment blanket group – 5 tubes intramammary ceftiofur, 24 hours apart. Treatment selective group - 2 tubes intramammary cephapirin sodium, 12 hours apart |
STCM = selective treatment of clinical mastitis
Lago et al has published two papers based on a large scale randomised clinical trial. The first paper evaluated short term clinical and bacteriological outcomes (Lago et al, 2011a), while the second followed up to present longer-term outcomes such as milk production and cow survival (Lago et al, 2011b). This was a multi-state, multi-herd clinical trial in the US evaluating 449 quarters, which were randomly assigned to either a blanket treatment or a selective treatment group. The proportion of animals treated based on a positive result for Gram-positive bacteria varied between herds from 31% to 89%, with the overall treatment risk being 51%. The authors found no statistically significant differences between cases in the blanket therapy group compared with the selective treatment group in days to clinical cure (2.7 vs 3.2 days), bacteriological cure risk within 21 days (71% vs 60%), new intramammary infection risk within 21 days (50% vs 50%) and treatment failure risk (81% vs 78%). In the longer-term outcome follow up, the authors found no significant differences in the same two groups between risk and days for recurrence of clinical mastitis (35%, 78 days vs 43%, 82 days), linear somatic cell count (4.2 vs 4.4), daily milk production (30.0 vs 30.7kg), risk and days for culling or death for the remainder of the lactation (28%, 160 days vs 32%, 137 days). The group therefore concluded that there were no differences in short- and long-term outcomes in the selective treatment group versus the blanket group, but that antibiotic use was reduced by approximately one half through the use of a selective treatment strategy. It should be noted that the authors did present a power analysis for the work and at 95% confidence and 80% power they would only detect differences in days to clinical cure of 1 day or more, treatment failure risk of 13% or greater and bacteriological cure risk of 17% or greater as examples. Therefore, while this work is representative of comparable treatment protocols to a UK situation, the size of the study would only detect larger differences between the groups than would be desirable in an on-farm situation and is possibly more valuable as part of the non-inferiority approach in the meta-analysis discussed in the previous section. The study carried out by Vasquez et al (2017) also found no significant differences between a blanket and selective treatment group based on positive identification of Gram-positive bacteria for days to clinical cure, post-event milk production, linear somatic cell count and 30-day survival. The selective treatment group on this single herd had 32% of quarters treated. It should be noted that the treatments in the two groups on this herd were significantly different, with the blanket group receiving 5 days of intramammary treatment of ceftiofur and the selective group receiving 1 day treatment of intra-mammary cephapirin. While this makes it more difficult to draw comparisons between the groups, it is interesting that there were no differences found despite the shorter treatment duration with a different active alongside the selective decision making.
Have you secured your CPD for 2024?
Join our programme of CPD courses, ‘Feeding the Dairy Herd’, to gain a comprehensive knowledge of dairy nutrition based on the most recent research available.
Upcoming courses include:
- Fundamentals of Dairy Cow Nutrition & Ration Formulation
- Dry Cow Feeding and Management
- Practical Feeding Management and Finances
Book your place at totaldairy.com
Proposed practical approach to starting a selective treatment of clinical mastitis protocol on farm
Step 1
Determine herd suitability for the selective treatment of clinical mastitis using farm data and information (Figure 1):

Mastitis pattern analysis eg AHDB QuarterPro
This will aid understanding of the mastitis seasonality, spread and pattern on a farm. The analysis must include both clinical mastitis and subclinical mastitis data to be comprehensive and complete. Contagious patterns are more likely to be driven by a high incidence of Gram-positive pathogens and therefore selective treatment of clinical mastitis will be focussed on targeted Gram-positive therapy rather than antibiotic reduction. Where the pattern suggests dry period origin, then the focus of attention for both prevention and treatment should be the factors contributing to the dry period infection rate. Environmental lactation mastitis patterns are most likely to be suited to selective treatment of clinical mastitis; however, the other factors below should be considered alongside the pattern analysis.
Clinical mastitis data
Good and accurate clinical mastitis data, ideally at the quarter level, is fundamental to monitoring mastitis treatment success and therefore ensuring that any changes made to testing or treatment protocols are beneficial rather than detrimental to cow health.
Bacteriology of clinical mastitis samples
A representative group of samples should be tested using bacteriology to determine the bacterial aetiology of clinical mastitis on each individual farm, particularly of mild and moderate cases when determining herd suitability. Herds where there is an unusual mix of pathogens or where less common mastitis pathogens are found, for example Klebsiella spp, Serratia spp or Mycoplasma spp, would not be suitable candidates for selective treatment of clinical mastitis because specific preventative measures and/or tailored treatment programmes are required to deal with these pathogens on farm.
Step 2
All farms:
Mastitis prevention
- All farms should focus on mastitis prevention with their vet using the data and information available.
Suitable farms:
- Selective treatment of clinical mastitis can be carried out under close veterinary supervision and monitoring of treatment outcomes.
Identify clinical mastitis case:
- Mild/moderate cases – administer non-steroidal anti-inflammatory drug and collect milk sample for testing
- Severe cases – treat immediately, administer non-steroidal anti-inflammatory drug + antibiotic if indicated + supportive therapy
- Run the mastitis pathogen identification test: tests should be rapid and accurate (de Jong et al, 2023b).
Based on the outcome of the test, a bespoke targeted treatment protocol should be written for each farm. The treatment options for each farm should be based on the farm data and information that was evaluated in step one (Figure 2).
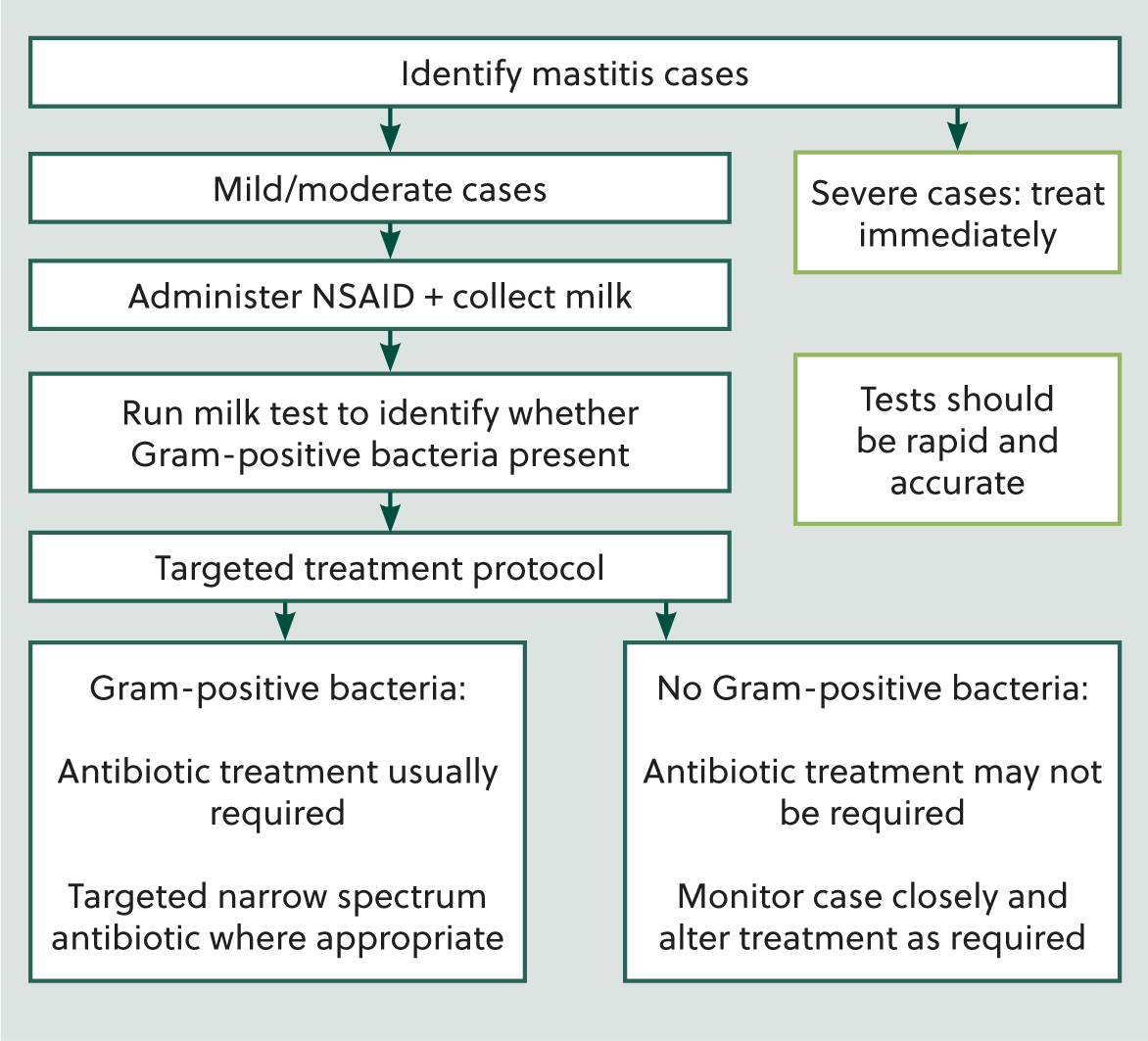
Example
Gram-positive bacteria identified: antibiotic treatment is usually required, a targeted (narrow spectrum) antibiotic can be used where appropriate. An additional step at this point may be to identify cows that are less likely to have a high chance of treatment success and base a separate treatment or culling decision on these factors, eg those with a previous history of clinical mastitis or high somatic cell count, higher parity animals.
No Gram-positive bacteria identified: antibiotic treatment may not be required, the case should be monitored closely and the treatment plan altered if there is worsening or no improvement to clinical signs.
Within the scientific literature (Lago et al, 2011a), failure to respond to treatment may be defined as:
- Increase in severity of case within 24–48 hours of initial treatment decision making OR
- Failure to decrease in severity (grade) of mastitis when assessed 48 hours after the initial treatment decision making.
Step 3
Regularly monitoring the new infection rate and treatment success and cure rates to maximise the chances of a successful outcome on the farm. Ensure that all clinical cases are recorded, including those that do not receive antibiotic treatment.
Conclusions
The selective treatment of clinical mastitis can be undertaken on farms meeting the appropriate selection criteria without compromising on cow health and welfare. The focus on all farms should always be on mastitis prevention; however, tailoring mastitis treatment protocols and incorporating cow-side testing can improve treatment success and maximise the chances of a successful cure. This article has presented a practical approach to selecting farms and creating a targeted treatment protocol for use on farm. This approach may reduce antibiotic use on farm without compromising udder and cow health and welfare.
KEY POINTS
- Selective treatment of clinical mastitis is the decision making around treatment of a mild or moderate clinical mastitis case based on the results of cow-side pathogen testing.
- Gram-negative pathogens often have a high self-cure rate and on some farms treatment with antibiotics may not be required.
- Gram-positive pathogens can be treated with a targeted narrow spectrum intramammary antibiotic.
- All mastitis cases will benefit from non-steroidal anti-inflammatory therapy at the onset of clinical signs.
- Devising farm-specific selective treatment protocols facilitates mastitis discussions on farm, a focus on preventative measures is always beneficial and can emphasise the importance of a whole herd approach to mastitis control.


