Infections caused by Neospora caninum were first described relatively recently, having first been identified in dogs in 1984 (Bjerkås et al, 1984), differentiated from Toxoplasma gondii in 1988 (Dubey et al, 2007) and identified in dairy cattle in New Mexico in 1989 (Thilsted et al, 1989). During the past 30 years, neosporosis has become well established as an endemic disease of cattle in many countries and a significant cause of abortion in cattle. It is an obligate intracellular protozoal parasite, which relies on both intermediate and definitive hosts to complete its lifecycle. The impact of neosporosis on health, welfare and economics of farming systems is being recognised via control programmes implemented by milk suppliers (Sainsbury Plc, 2021) and farm assurance schemes (Red Tractor, 2022).
Clinical signs
The main clinical presentation in Neospora-positive cattle is abortion from 5–7 months of gestation and can occur as early as 4 months (McAllister, 2016). Seropositive cows are three to thirteen times more likely to abort without any concurrent signs in the dams (Hall et al, 2005; Wouda et al, 1998). Aborted fetuses can be fresh, autolysed or mummified (Figure 1), depending on the time between fetal death and expulsion of fetal material (McAllister, 2016). If calves are born alive, they may be clinically normal, latently infected (but apparently normal) or have obvious defects. These defects can include being born prematurely, noticeably small, ataxic, recumbent, having poor conscious proprioception and/or reduced patellar reflexes (Figure 2) (McAllister, 2016). Dams may subsequently suffer from retained fetal membranes or metritis, as they would with any abortion.


Prevalence
The estimated prevalence of neosporosis in the European cattle population is around 15% (95% confidence interval 12–18%) (Ribeiro et al, 2019) and infection is more common in dairy cattle than beef cattle (Ribeiro et al, 2019). The risk of Neospora seropositivity increases with the presence of farm dogs and with the number of dogs (Ghalmi et al, 2012; Imre et al, 2012). Younger dogs are more often associated with oocyst shedding, but oocysts have been identified in dogs from 2 months to 13 years of age (Schares et al, 2005; Paradies et al, 2007). Recently acquired dogs are also associated with a higher risk of infection as gut immunity builds up with repeated ingestion that prevents continued shedding (Dijkstra et al, 2001).
Of the tests carried out by the author's practice in the south of England in 2017, 3.9% of cattle sampled for monitoring purposes tested positive for Neospora caninum antibodies, compared to 17.6% sampled for diagnostic purposes when there was a suspicion of disease (Table 1). The total seroprevalence for the cattle sampled was 6.2%, which is likely to be low because farmers monitoring herds for disease might be carrying out active eradication programmes. These findings reflect seroprevalence estimates from the late 1990s (18% of aborting cattle seropositive compared to 6% in the control group), as well as more recent research (18% of aborted fetuses were polymerase chain reaction [PCR] positive) (Davison et al, 1999a; Bartley et al, 2019) suggesting little progress has been made towards eliminating the disease in the UK.
Table 1. Test types and results for Neospora caninum testing in 2017 in a practice in the south of England
| Test type | Negative ELISA | Positive ELISA | Total |
|---|---|---|---|
| Diagnostic | 61 | 13 | 74 |
| Monitoring | 346 | 14 | 360 |
| Total | 407 | 27 | 434 |
ELISA = enzyme-linked immunosorbent assay
Pathogenesis
Neospora caninum has a facultative heteroxenous lifecycle (it can use more than one host during the lifecycle) (Dubey et al, 2006). The definitive hosts are dogs and other canids, potentially including foxes (Reiterová et al, 2016; Wapenaar et al, 2006). Dogs shed unsporulated oocysts in their faeces which take around 24 hours to sporulate into the infective form of two sporocysts, each containing four sporozoites. If these are ingested by cattle or other intermediate hosts, such as rats, sheep, goats and deer (Jenkins et al, 2007), the sporozoites rapidly differentiate into tachyzoites that invade the epithelial cells of the gastrointestinal tract. This asexual replication (endodyogeny) causes cell lysis (McAllister, 2016). Phagocytosis of these tachyzoites by the host immune system allows spread around the body and invasion of neurones, vascular endothelial cells, myocytes, hepatocytes, renal cells, alveolar macrophages and placental trophoblasts. Upon reaching these locations, bradyzoites develop slowly and become encysted. Latent bradyzoites may quiesce during pregnancy and migrate via the placenta to the fetus, or if infection occurs during pregnancy tachyzoites can cross the placenta (Williams et al, 2009). Tachyzoites that have travelled to the fetus in-utero have a predilection for the brain and spinal cord. The cycle is concluded with canids ingesting bradyzoite-infected fetal neural material or placenta. Bradyzoites can survive for up to 2 weeks at 4°C but will be killed by freezing (Dubey et al, 2007). In the definitive canine host, bradyzoites become schizonts; these release merozoites which become gametes and undergo sexual reproduction to produce unsporulated oocysts, which are shed in the faeces.
Transmission
Transmission can occur horizontally – resulting in latency or exogenous transplacental infection – or vertically (endogenous transplacental infection) (Figure 3) (McAllister, 2016). The efficiency with which N. caninum is transmitted transplacentally has been demonstrated numerous times in the literature since the identification of the parasite (Björkman et al, 1996; Bergeron et al, 2001; Hall et al, 2005). The transmission of disease in canines (also horizontal or vertical) is beyond the scope of this article, which will focus on routes of bovine transmission. Lateral transmission via ingestion of oocysts can occur when the cow is pregnant, which may result in exogenous transplacental transmission if oocysts also cross the placenta (Trees et al, 2005), or when the cow is not pregnant resulting in latent infection. If infection occurs after the first trimester of pregnancy there is improved fetal immunocompetence, meaning it is more likely that the pregnancy will result in the birth of a latently infected calf rather than abortion (Dubey et al, 2006).
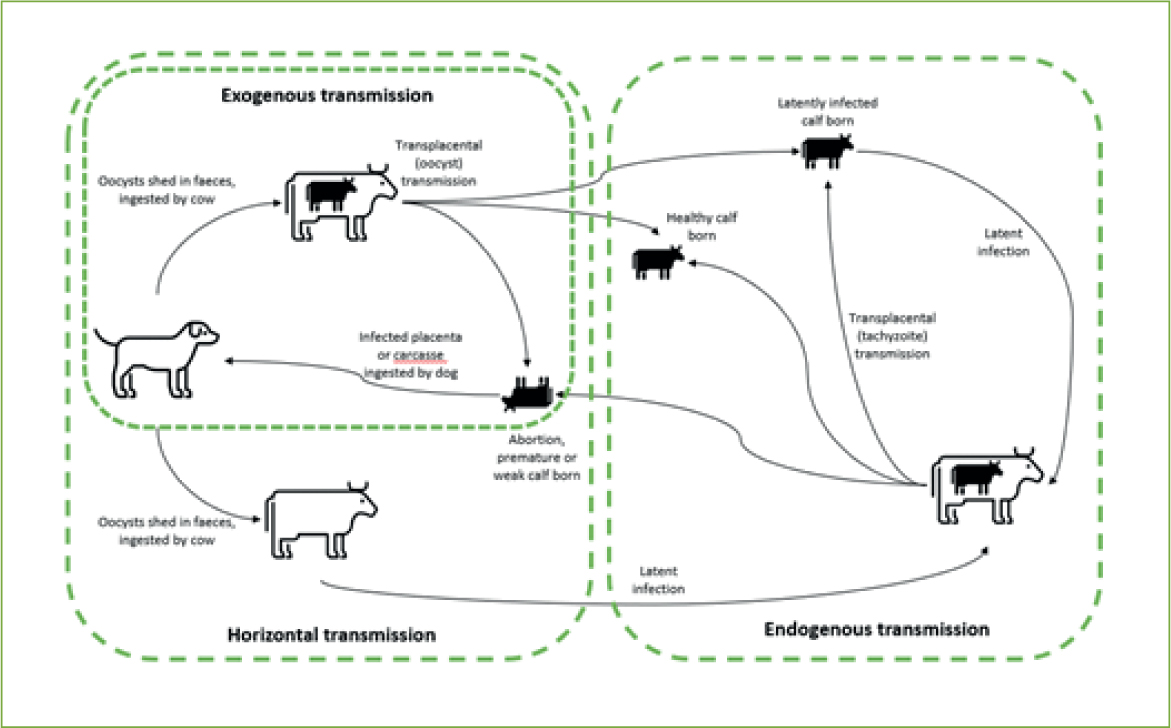
When a latently infected cow later falls pregnant, the organism can recrudesce and cross the placenta (endogenous transmission). Cattle do not transmit antibodies transplacentally, which means that seropositive calves must have synthesised antibodies to the organism in utero (Dubey et al, 2007). Seropositive latently infected cattle will remain clinically normal but produce congenitally infected calves that may be aborted before reaching full term (Anderson et al, 1997). This may occur in several pregnancies in the same dam, although there is some evidence the risk might reduce over subsequent pregnancies and that transmission rates are around 95% (Anderson et al, 1995; Davison et al, 1999b; Dijkstra et al, 2003). This suggests horizontal transmission is an important means to perpetuate long-term infection within a herd, because vertical transmission does not reach 100%. Infection does not appear to spread venereally, either by natural or artificial insemination (Serrano et al, 2006; Ferre et al, 2005). There is also no evidence of cow-to-cow transmission or lactogenic transmission from cow to calf (Dubey et al, 2007).
Epidemic and endemic patterns
Neosporosis is described as epidemic when the abortion out-break is temporary and if 10–15% of cows abort within 4–8 weeks (Schares et al, 2002). The pattern is described as endemic if it persists in the herd for several months or years. Epidemic out-breaks at times of immunosuppression can subsequently result in endemically affected herds. These patterns link to the types of transmission detailed above. The epidemic pattern is associated with horizontal infection of naïve pregnant dams (McAllister et al, 2000), especially where many pregnant cows are infected at the same time (Schares et al, 2002) with the severity of the outbreak and likelihood of abortion associated with infective dose, pathogenicity of strain or dam susceptibility (Gondim et al, 2004; Dijkstra et al, 2008). The time from horizontal infection to abortion is 17–33 days (Andrianarivo et al, 2000; Williams et al, 2009).
Endemic patterns are associated with endogenous placental transmission, where infection recrudesces during pregnancy. The reasons for recrudescence are poorly understood (Dubey et al, 2007) but may be linked to ingestion of toxic feeds, concurrent disease or other reasons for reduced immunity (Bartels et al, 1999; Bech-Sàbat et al, 2007).
Diagnosis
The diagnosis of bovine abortion can be frustrating and neosporosis is no different. A seropositive cow does not definitively mean that the abortion was caused by the Neospora infection. Farm or cow history is an important starting point for diagnosis of any abortion: are the aborting cows systemically healthy, is there a history of neosporosis on the farm, what is the pattern of abortion? This knowledge can help guide the approach to testing and where to focus the effort and economics.
Testing can be carried out using serology (immunofluorescence antibody test [IFAT] or antibody enzyme-linked immunosorbent assay [ELISA]) or direct testing (immunohistochemistry [IHC] or PCR). Direct testing on the fetus will usually occur as part of a general enquiry into abortion, but individual cow ELISA testing may be carried out on farms with a known history of neosporosis, either as part of a monitoring system or where a cow is found to have aborted.
If cows are tested near the time of abortion (rather than being identified as empty later on) the affected dams are likely to have high serological titres (McAllister, 2016). IFAT is considered to be highly sensitive and specific; it can be used on maternal blood and fetal fluids if the abortion occurs after 5 months of gestation (Wouda et al, 1997). While serology testing only informs of prior/latent infection, a suitably high titre may be sufficient for a presumptive diagnosis – McAllister (2016) considers an IFAT result of 1:1600 as ‘abortion likely to be caused by Neospora’ and 1:6400 as ‘highly probable’. Interpreting results should therefore be more refined than a simple negative or positive. In a herd with endemic neosporosis there will be seropositive animals that have not aborted because, as discussed earlier, not every positive dam will abort. In the UK, most commercial serological testing uses antibody ELISAs that can be carried out on bulk milk to determine herd-level infection as well as on individual blood or milk samples.
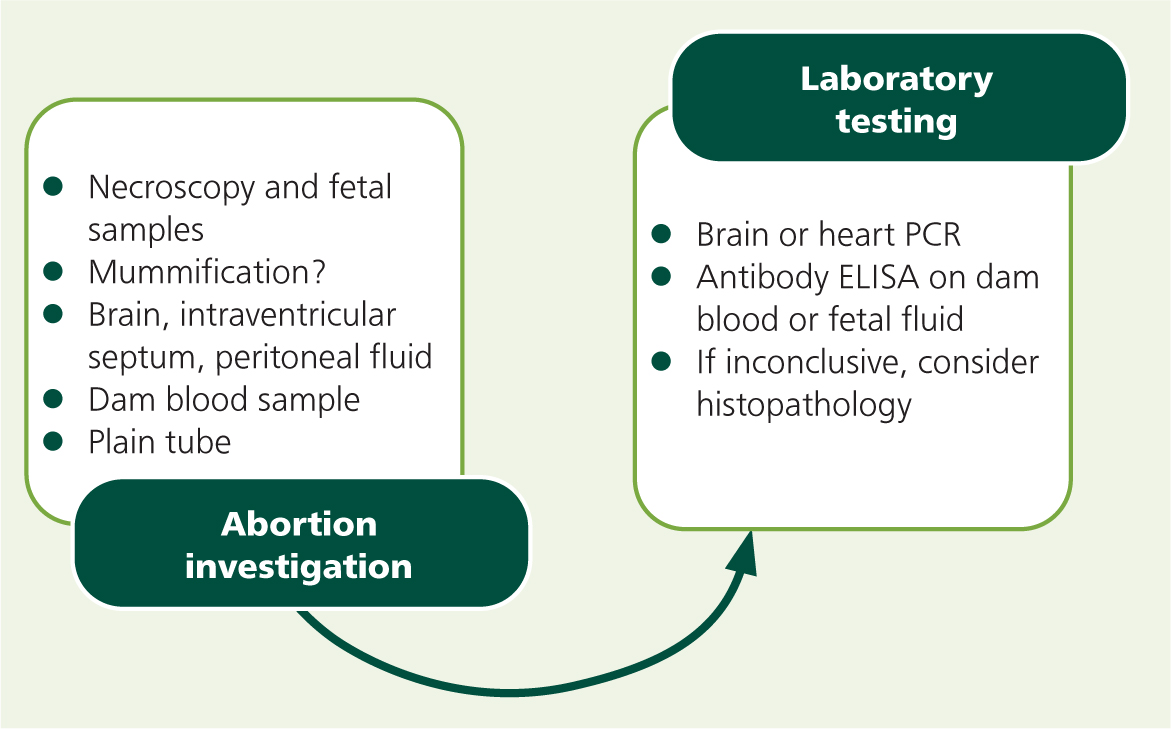
Examining aborted fetuses may provide a definitive diagnosis, but it is important to warn farmers that sporadic abortions often do not provide a conclusive result. Necroscopy of the fetus is often not diagnostic, but mummification can suggest neosporosis (McAllister, 2016). Autolysis may preclude useful testing, but samples to take would include (in order of usefulness): brain, heart (intraventricular septum), liver, spleen, skeletal muscles, and placenta as well as peritoneal fluid for fetal serology (Henning et al, 2002; Dubey et al, 2006). Histopathology can detect multifocal inflammation (encephalitis, myocarditis, periportal hepatitis) and necrosis of a variety of tissues, as well as the presence of tachyzoites in the fetal brain (Wouda et al, 1997). PCR of brain tissue is highly sensitive and specific, whereas IHC is highly specific but poorly sensitive, with brain IHC being more sensitive than heart or liver testing (Sager et al, 2001; Wouda et al, 1997). The author prefers to obtain all the above samples and then request testing in a step-wise manner when investigating – initially requesting brainstem (or heart) PCR where neosporosis is a likely differential diagnosis (Figure 4).
Control and prevention
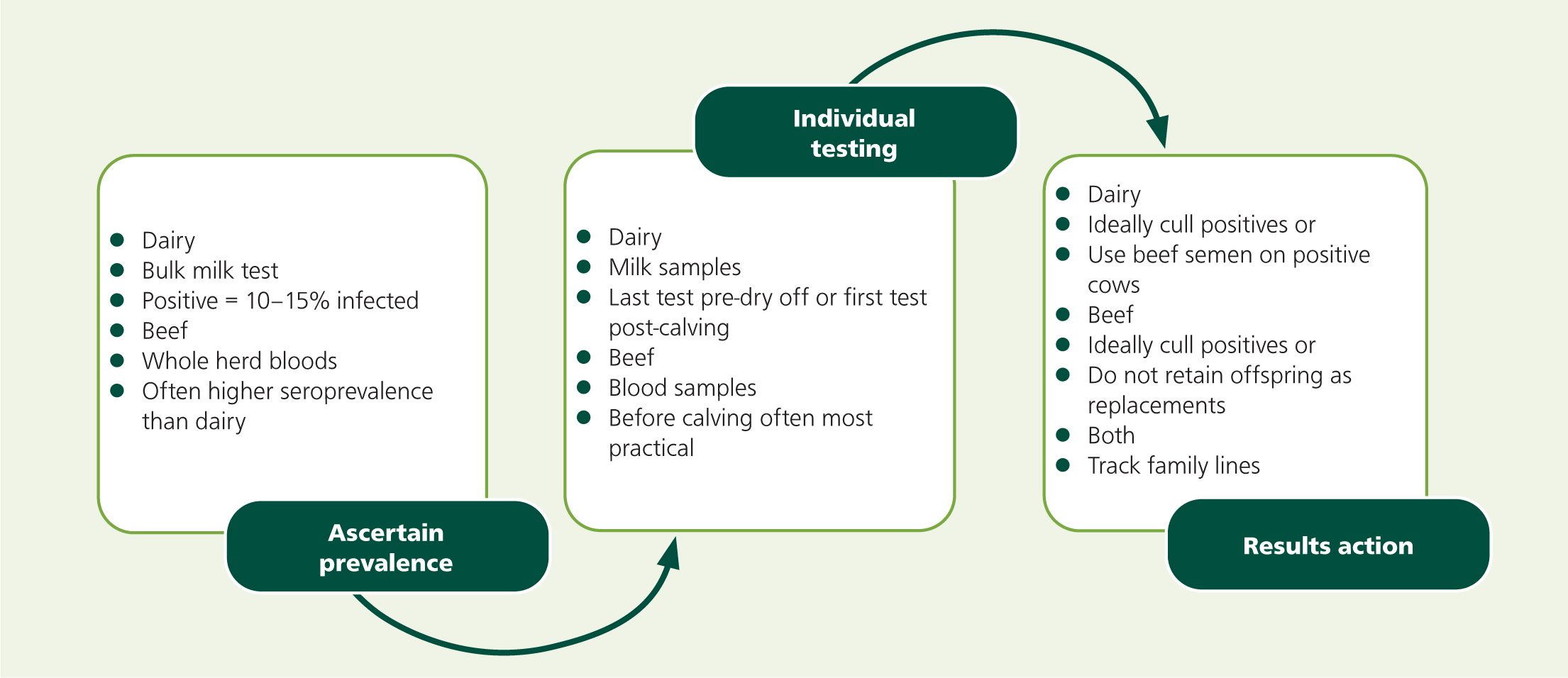
If a producer is concerned that they may have a problem with neosporosis, the first step is identifying the level of disease on the farm. It is likely that an abortion enquiry has yielded a positive result to justify embarking on further testing or that the farm is interested in joining an accreditation scheme. A sensible starting point for dairy units is testing bulk milk using an ELISA – the result will be positive if 10–15% of the herd are seropositive (Björkman and Uggla, 1999). For testing individual dairy cows, the author advises that cows are tested at the last milk sampling before drying off to increase the chance of seropositivity, as titres increase during gestation (Piergili Fioretti et al, 2003; Nogareda et al, 2007). If farmers want to make breeding decisions before service, a small study suggested that titres remain high for up to 3–4 months after calving (Piergili Fioretti et al, 2003). Pre-colostral ELISA testing of heifer calves has been reported experimentally (Andrianarivo et al, 2005) but is unlikely to be effective practically as few farmers will be able to blood sample calves before colostrum intakes. For beef herds that calve in a block, a whole herd screen is the best approach. Targeting a time either in late gestation or before the next service period begins can increase the chance of finding seropositive cows (Piergili Fioretti et al, 2003; Nogareda et al, 2007). Possible steps to take when attempting to eradicate neosporosis from a farm are shown in Figure 5.
Once Neospora infection has been identified within a herd, eradication can be a challenge because of infected family lines and a lack of an available effective vaccination. In dairy herds, it has been suggested that beef semen can significantly reduce the risk of abortion. Supporting factors for this include fewer brain lesions, reduced transplacental transfer of the parasite or improved placental function demonstrated by higher pregnancy-associate glycoprotein levels (De Meerscham et al, 2002; Almería et al, 2009). The use of beef semen also avoids the disease perpetuating through replacement heifers and means that cows are more likely to reach their next lactation (Dubey et al, 2007). Beef cows are less likely to abort than dairy cattle (De Meerscham et al, 2002) which means that vertical transmission will infect many generations of cows and result in high intra-herd seroprevalence. These beef breeds will still be more likely to abort than healthy cows and so the economic losses can be significant.
To effectively remove disease from either beef or dairy farms, seropositive animals should be removed from the herd – a test and cull approach (French et al, 1999). If seroprevalence is high it may not be economically viable to achieve this rapidly because it would involve a high culling rate. Retaining seropositive animals but not breeding replacements from them, or culling when they experience fertility problems, might be required in this situation. Identifying infected family lines can speed up the eradication process because female calves of infected dams are likely to also be seropositive. The author undertakes this analysis on a number of dairy farms with known disease issues (Table 2, Figure 6). Seropositive, vertically infected heifers may appear seronegative when tested pre-breeding at around 13–14 months of age, in comparison to younger heifers or older pregnant heifers (Davison et al, 1999). Therefore, heifers should be tested at less than a year old or females tracked as described above.
Table 2. An example of Neospora tracking on a dairy farm demonstrating positive cows, their dams, offspring, and presence in the herd
| Cow ID | Test date | Result | Cow still in herd? | Dam | Still in herd? | Off-spring 1 | Date of birth | Off-spring 2 | Date of birth | Siblings | Date of birth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | April 2021 | Positive | N | 119 | N | 272 | 2020 | ||||
| 38 | July 2021 | Positive | Y | 254 | N (fertility issues) | ||||||
| 159 | July 2021 | Positive | Y - Barren | 201 | N (fertility issues) | 145 | 2019 | 169 | 2018 | 160 | 2019 |
| 145 | September 2021 | Positive | Y | 159 | Y |

Definitive host infection must also be considered – seropositive dogs may be less likely to shed oocysts if they were infected some time ago as the oocyst shedding time period is a few weeks only (McAllister, 2016). In the UK, dogs are seldom tested either for seropositivity or fecal oocyst shedding. All dogs – particularly those more likely to be seronegative (young dogs) should be kept away from calving areas. Cows should not calve in fields that contain footpaths, as this will reduce the risk of the passing dogs being able to ingest aborted or placental material.
Once a herd is free of disease, farm biosecurity is essential. If new stock is purchased, they should be obtained from accredited seronegative herds or tested before breeding. Dog walkers are often considered to increase the risk of neosporosis spread. Signage (Figures 7 and 8) can be used to encourage dog walkers to remove faeces from pasture and it is advisable to avoid feeding pregnant cattle silage from well-known dog walking fields to avoid epidemic outbreaks (Schares et al, 2002). Rapidly removing aborted or calving material is another way to reduce environmental exposure (French et al, 1999).
Conclusions
Neosporosis is an important and significant cause of abortion in UK beef and dairy cattle herds. The disease is caused by N. caninum, which is an intracellular protozoal parasite that has a facultative heteroxenous lifecycle. Dogs are definitive hosts and cattle are intermediate hosts; cattle can be infected via vertical or horizontal transmission. Neosporosis causes abortion in cattle, although seropositive cows appear normal and may give birth to live offspring. Diagnosis is made by direct methods on fetal material or by serological testing. Control is challenging because of a lack of vaccination and latent infection meaning that infected cattle remain an ongoing risk for perpetuating disease. A test and cull approach will lead to a neosporosis-free status, but will take time because seropositive animals may display low serological titres when tested at times not near to calving and farms with high levels of infection will be unable to immediately cull all positive cattle for economic reasons. Further research into the reasons for latent infection recrudescence, and vaccination and antiprotozoal treatments will add to the understanding of this disease and improve eradication strategies.

KEY POINTS
- Neospora caninum is an obligate intracellular protozoal parasite that relies on intermediate and definitive hosts to complete its lifecycle.
- Neospora infection leaves cattle three to thirteen times more likely to abort than a healthy animal; calves may be born alive but be latently infected or have obvious defects.
- Disease transmission can be horizontal, when cattle ingest feed contaminated with infected dog faeces, resulting in latency or exogenous transplacental infection, or vertical when latent infection recrudesces producing a congenitally infected calf.
- Testing involves serology or direct testing if abortion material is available; serology can provide a presumptive diagnosis when carried out close to an abortion event.
- Disease eradication takes time and involves identifying infected cows and infected family lines via serology before removing these individuals from the herd.


