Mycoplasma bovis was first recognised as a bovine pathogen in 1961 (Hale et al, 1962) and in the UK in the 1970s (Davies and Broughton, 1976) and is one of 13 Mycoplasma spp. known to infect cattle. The significance of mixed Mycoplasma infections in cattle populations will vary depending on scenario with some Mycoplasma species, such as M. bovis and Mycoplasma dispar, considered more pathogenic than, for example, Mycoplasma bovirhinis (Nicholas and Ayling, 2003).
Mycoplasma spp. have no cell wall and instead have a cell membrane with variable surface lipoproteins. These are used for organism attachment and elicit variable immune responses from the host. The organism can also produce and survive in biofilms and it is this plus variable evasion of the host's immune response which permits the organism to persist and cause disease (Maunsell et al, 2011).
Therapeutics and treatment options
Some of the organism's characteristics have a direct effect on antibiotic treatment choices. As the organism has no cell wall B lactam antibiotics are ineffective. The organism does not synthesise folic acid and therefore sulphonamides are ineffective. M. bovis tends to be susceptible to antibiotics that interfere with protein or DNA synthesis such as the tetracyclines, macrolides, lincosamides, florfenicol and fluoroquinolones (Taylor-Robinson et al, 1997).
Readily available and cheap antibiotic sensitivity testing techniques such as disk diffusion are not appropriate for Mycoplasma spp. Therefore, currently assessment of antibiotic sensitivity patterns for M. bovis isolates cannot easily be carried out in diagnostic labs or veterinary practices. Minimum inhibitory concentration (MIC) testing is available for M. bovis and, although more expensive, is justifiable in practice to ensure appropriate and responsible antibiotic selections.
A study comparing MIC values for 45 M. bovis isolates identified between 2004 and 2009 showed increases in MIC values for chloramphenicol, oxytetracycline and the fluoroquinolones suggesting developing resistance (Ayling et al, 2014).
Mycoplasma bovis causes several clinical disease presentations in UK cattle
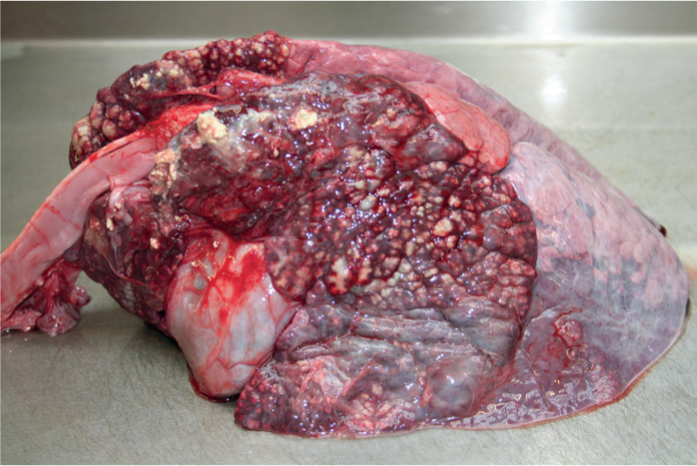
Pneumonia: This is the most common clinical disease presentation (Figure 1). M. bovis can cause disease as a sole pathogen, or more commonly in combination with other pathogenic respiratory bacteria, viruses or parasites. Disease can occur in all ages of cattle in both beef and dairy systems. A pathology finding of caseonecrotic pneumonia is recognised commonly in dairy calves and in some of these cases response to treatment is poor, with relapses commonly reported.
Mastitis: M. bovis mastitis is not common in the UK dairy herd and the organism can also be detected as an incidental finding in milk. However, outbreaks of mastitis in individual herds can be severe and sometimes occur in association with arthritis. M. bovis is considered a contagious mastitis pathogen and response to treatment is very poor.
Arthritis/synovitis: This can occur in all ages of cattle and outbreaks have occurred. One or more lower limb joints are usually affected and severe cases can be refractory to treatment. It is suspected that lower grade and sporadic cases of M. bovis arthritis are occurring in herds and this may be under-diagnosed.
Otitis media: This clinical presentation is seen most commonly in youngstock, particularly young dairy calves which present with an ear droop or head tilt. Reports from veterinary practitioners would suggest that this presentation has been seen much more commonly in the last 10 years.
Other disease presentations: These occur less commonly and include keratoconjunctivitis, meningitis, infertility and abortion.
Diagnostics
Pathogen detection can be achieved by molecular methods, polymerase chain reaction or diffuse gradient gel electrophoresis (DGGE). DGGE gives an advantage of being able to detect DNA from all potential Mycoplasma spp. Mycoplasma culture methods are also available and an isolate is required if assessing antibiotic sensitivity or autogenous vaccine production is being considered. The pathogen detection method has to be considered based on a combination of test cost and reasons for testing and can be applied to milk, lung, swabs, joint fluid etc. One of the challenges to culture methods is bacterial overgrowth, and sampling using Mycoplasma transport media is suggested to reduce these risks.
Serological testing can be of use as part of herd health surveillance and health planning. Seroconversion occurs after 4 weeks. Although this does not prove a direct association with a disease outbreak, it does confirm exposure and if the clinical picture on farm is consistent then this permits future health planning decisions.
Risk factors
Information on suspected risk factors for M. bovis infection and disease transmission are available and can help with control but are poorly evidence-based in the UK. An open herd policy, large and expanding herd size, unpasteurised cows' milk/waste milk feeding to calves, unhygienic milk feeding practices, automatic calf feeding systems, aerosol transmission, quality of housing/ventilation, and milking parlour spread are all considered potential risk factors for disease transmission.


