An incursion of Japanese encephalitis virus disease has been identified as commencing in North-eastern Australia from April 2021 (Carr, personal observation), resulting in prolonged gestation length, mummified and stillborn piglets, often with severe fetal abnormalities and congenital tremors (van Dissel et al, 2022). The disease has been recognised to have occurred on nearly 80 farms over an 18 months period with some seasonal impacts and large mosquito numbers afflicting farms. The virus is transmitted by mosquitos to pigs; there is possible transmission between pigs. There have been no obvious clinical signs seen in nursery to adult pigs (Australian Government Department of Health, 2022a).
The virus causing Japanese encephalitis is zoonotic and has caused clinical infection in 40 people (Australian Government Department of health, 2022), resulting in the death of at least five people. None of the people showing clinical signs had any contact with the pig industry. No one involved in the pig industry or students handling potentially infected tissues showed any clinical signs of the disease. There is no evidence of Japanese encephalitis virus transmission between pigs and people, only via mosquitoes.
The virus has been found in a number of water birds (Scherer et al, 1959; Soman et al, 1977), horses (Mackenzie et al, 2007) and an alpaca (Australian Government Department of Health, 2022b).
While this specific condition is unlikely to occur in the UK, there are other flaviviruses that could enter the UK and that could infect people, as well as various farm and wild species of animals, especially as climate changes occur.
Japanese encephalitis virus
Japanese encephalitis virus is an Flavivirus (International committee on Taxonomy of Viruses, 2017), an enveloped positive strand RNA virus endemic in eastern and south-eastern Asia (Williams et al, 2019). There are five strains of Japanese encephalitis virus recognised. Flaviviridae is a large group of viruses, often arthropod-borne. Japanese encephalitis virus genotype 3 has previously been detected in trapped insects and water birds in the northern tropical regions of Australia since 1995 (Mulvey et al, 2021). The current strain, however, is different, and identified as a type 4 which was recognised in 2021 in the Tiwi islands (Mulvey et al, 2021).
Japanese enchaphalitis virus is a zoonotic-mosquito borne pathogen, causing clinical signs in an estimated 70 000 people annually globally (Williams et al, 2019). The majority of infections are inapparent.
People and horses may become affected but are dead end hosts (Mackenzie et al, 2007). Waterbirds are the reservoirs and pigs may act as amplification hosts during their viraemic phase for 5 days (Ricklin et al, 2016). The virus is notifiable in Australia. Vaccination of people at risk is being carried out. It is noted that the vaccine is a type 3 Japanese encephalitis virus vaccine. Vaccination in pigs is not currently carried out in Australia. In south-east Asia, vaccination of pigs using a live attenuated vaccine to genotype 3 is commonly practiced, together with immunity stabilisation of gilts through feedback using materials produced at farrowing (Carr, personal observation).
Distribution in Australia
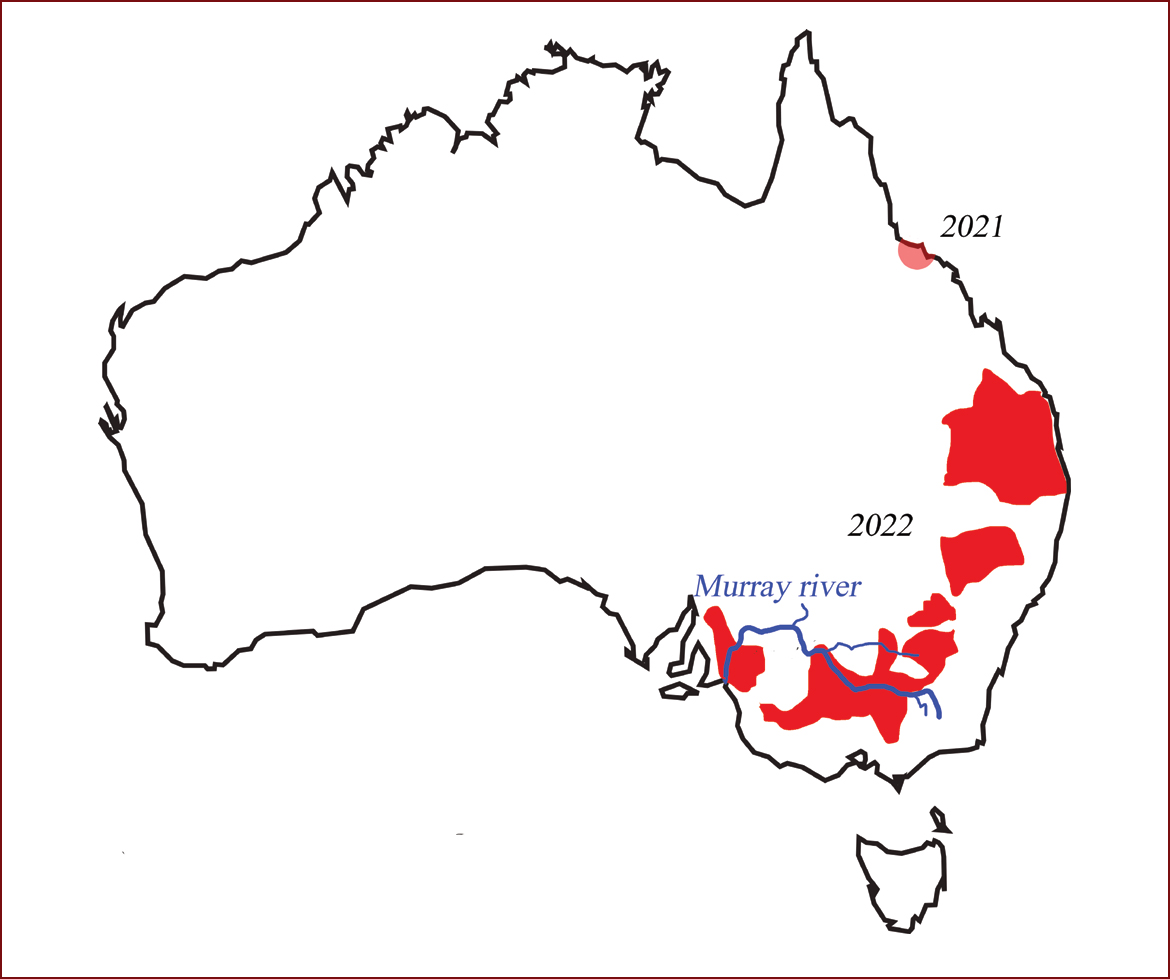
Clinical signs were first observed in April 2021 in northern Queensland, but in 2022 more cases were seen in the southern states on a number of properties. Samples from northern Queensland were initially negative to flaviviruses but when retested in June 2022 for Japanese encephalitis virus by polymerase chain reaction (PCR) they were found to be positive (Figure 1).
Case history presentation
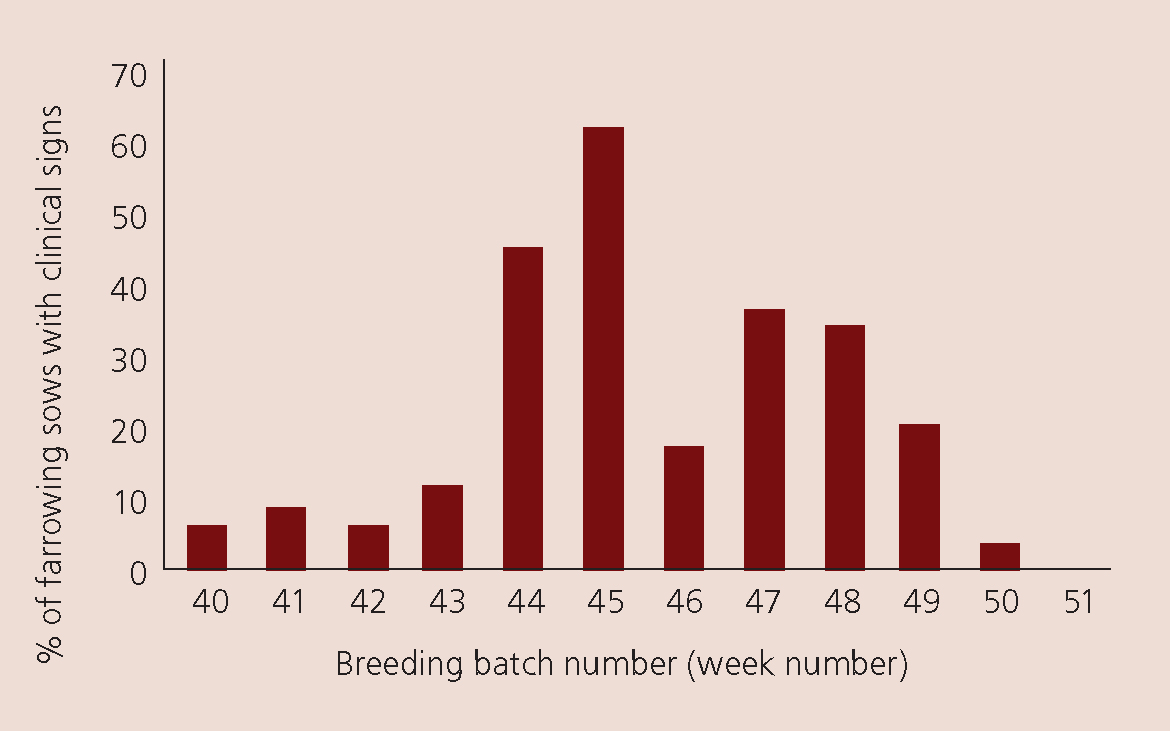
Figure 2 demonstrates clinical signs on a typical farm in southern Queensland. Clinical signs of an increase in born dead lasted 10 weeks. The farm is a batching system weaning 36 sows weekly. On another farm, of 6 sows a week in northern Queensland, after the initial clinical signs resolved future breeding production returned to normal (Figure 3). The large red marker in Figure 3 indicates when the results are ‘out of control’. Thus, in quarter 4 2021 the farm had a significant decrease in piglets born alive and increase in those born dead. The farm data returned to normal parameters the following quarter.
Clinical signs
As in any disease presentation there are a range of clinical signs. The clinical case presentations that should raise concern in veterinarians and the farm health team were:
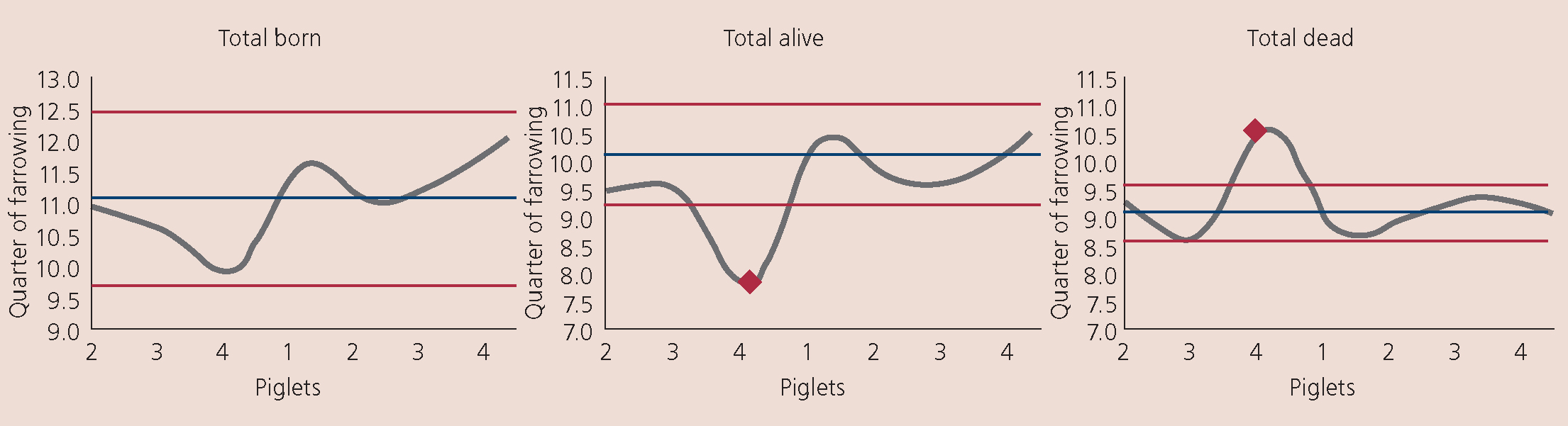
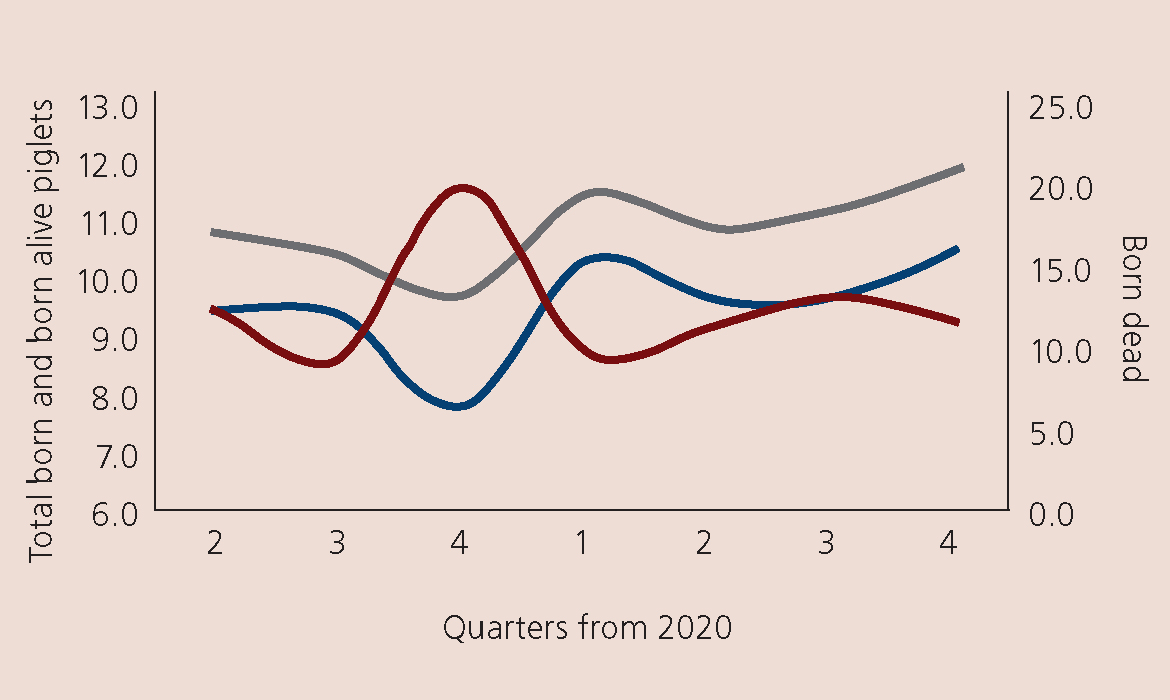
- Reproductive parameter changes (Figure 4)
- There was a significant increase in piglets born dead and a significant decrease in those born alive (Figure 4)
- There was no significant impact on batch farrowing rates. During the outbreak the average farrowing rate was 81%.
Temporary orchitis
There was temporary orchitis in a few boars with enlarged testicles.
Prolonged gestation length
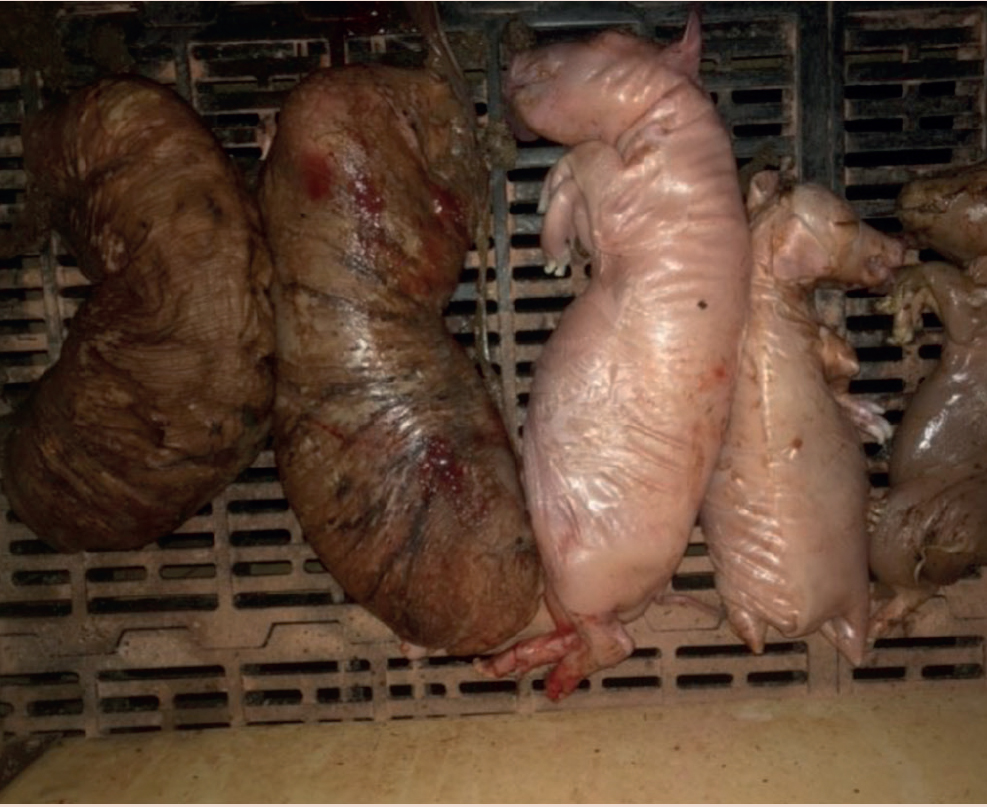
Sows failed to farrow by day 117 and were induced. At parturition they passed large numbers of mummified (Figure 5 and 6) and stillborn piglets. The clinical signs are seen in all parities of sows. It should be noted that the normal gestation length of Sus scrofa (wild boar) is 112 to 120 days. All the sows induced with longer than normal gestation had no live-born piglets.

Congenital abnormalities
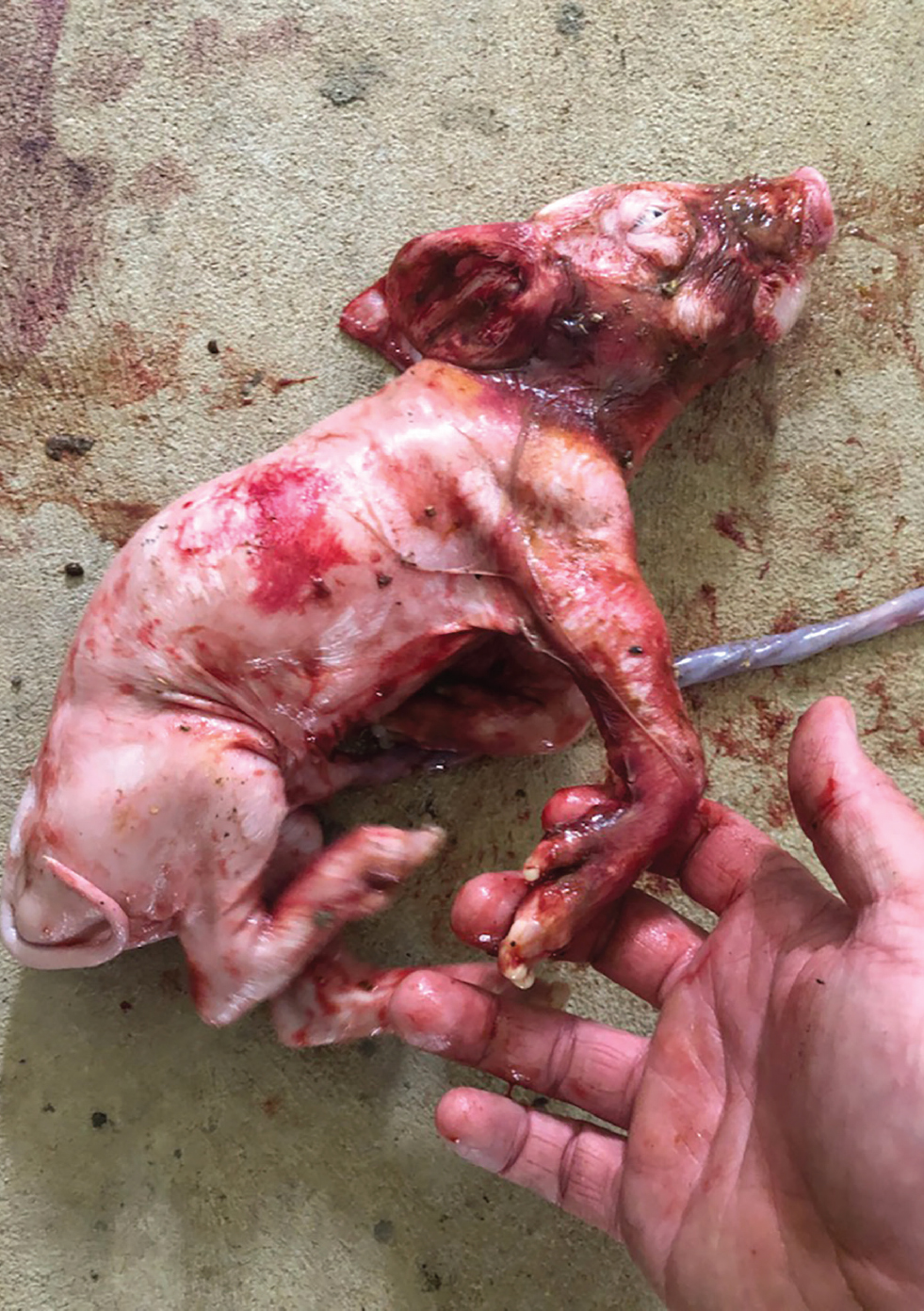
Stillborn piglets presented with an array of congenital abnormalities, including severe arthrogryposis and changes in limb proportions and in some brachygnathia. Some of these piglets were born alive. Figure 7 shows an affected piglet.
Neurological abnormalities
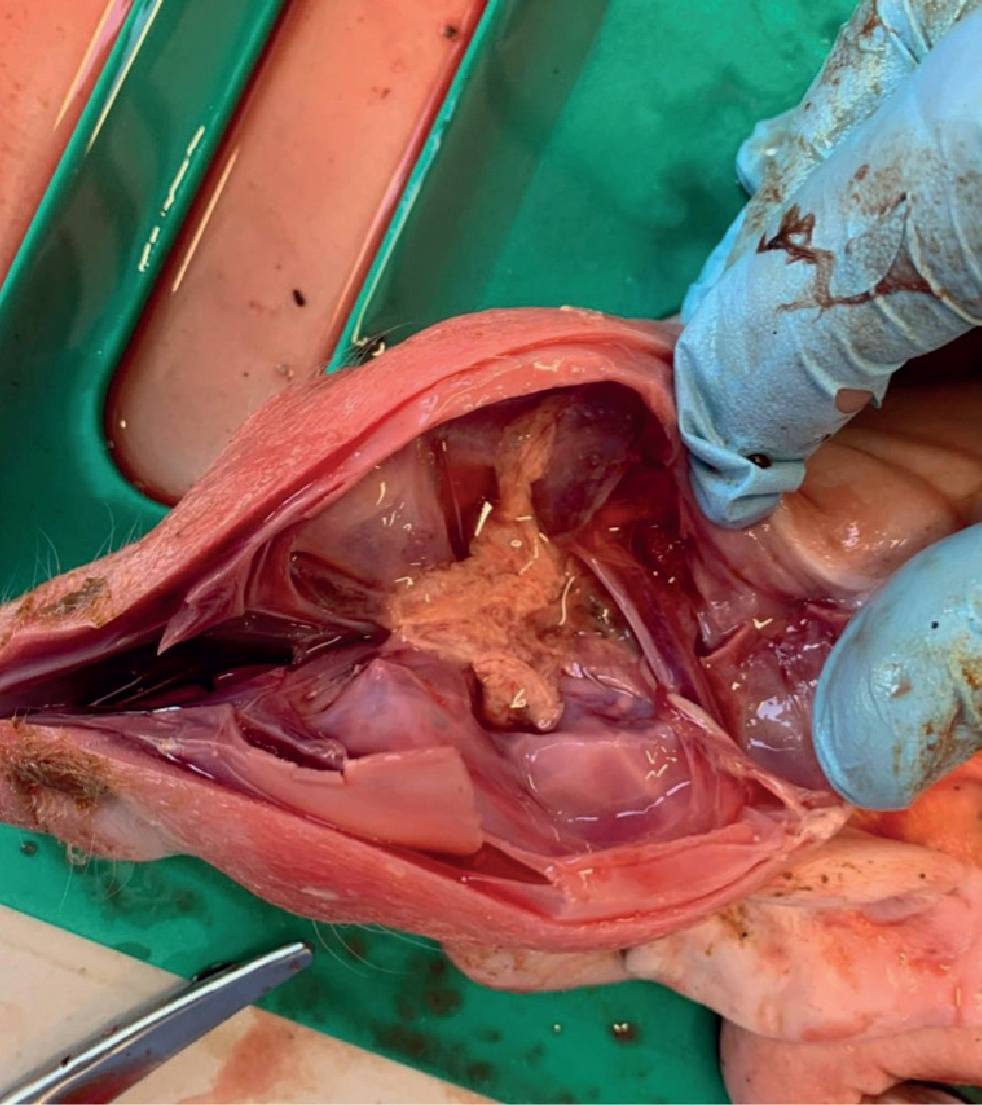
A striking finding can be cerebral and cerebella aplasia, hydranencephaly and meningocele (Figure 8).
Congenital tremor piglets
Some piglets were born alive exhibiting trembles.
Laboratory examination
The samples from piglets and sows were all negative for atypical congenital tremor; Aujeszky's disease; classical swine fever virus; African swine fever virus; menangle; influenza virus; Murray valley encephalitis virus (MVE); Kunjin virus and porcine reproductive and respiratory syndrome viurs. Sows with affected litters were polymerase chain reaction (PCR) negative to Japanese encephalitis virus but positive for antibodies to Japanese encephalitis virus, Kunjin and MVE, which are related viruses.
Japanese encephalitis virus was found in brain, pleural fluid and spleen of submitted stillborn piglets.
Control measures
Control measure are all based on controlling mosquitos (Table 1 and Figure 9). It is appreciated that the problems started in Australia several months earlier with minimal clinical signs. In addition, with winter starting in the southern hemisphere, mosquito numbers decline significantly and the clinical signs, while lasting 10–15 weeks ceased when the mosquito numbers dropped, on multiple different farms across south-eastern Australia.
Table 1. Possible mosquito control measures
| 1 | Maintain and repair all windows |
| 2 | Ensure no food enters the dung channel. Creep feed is an ideal medium for fly multiplication |
| 3 | Place a 2 mm mesh over the windows and air intakes. This must be kept clean to prevent the mesh reducing ventilation rates. This can be difficult |
| 4 | The use of air-filtration systems to reduce viral particles should be considered |
| 5 | The solid manure pile should be covered as soon as possible after tipping faeces and then properly compacted to generate heat which will kill the larvae |
| 6 | Remove faeces as often as possible |
| 7 | Remove afterbirths as soon as possible (immediately) |
| 8 | Have efficient pressure washing |
| 9 | Remove all fermenting food |
| 10 | Insecticides should never be used as a substitute for good hygiene. Care should be taken to ensure no unnecessary spillage of food. Ensure that a build-up of animal waste products in passageways or on ledges around pens is prevented |
| 11 | Flood slurry channels in summer with water to cool and break up slurry crust |
| 12 | Turn lights off at dusk and leave them off over night |
| 13 | Reduce weeds around buildings by 2 metres |
| 14 | Do not cut down any weeds within 3 weeks of slaughter |
| 15 | Apply larvicides to the manure and bait |
| 16 | Remove all rubbish around the farm, especially any water holding — old tyres, drinker's buckets etc |
| 17 | Regularly raise curtains to stop pooling of water in curtain material |
| 18 | Place light near the exit fan to help suck out flies |

Vaccination
Vaccination is normally with a live attenuated Japanese encephalitis virus vaccine. With this Australian strain being strain 4 and the government authorities relucant to import live vaccines, an Australia dedicated autogenous vaccine is being developed (Richards, 2022).
Discussion
While the clinical signs of Japanese encephalitis virus infection are recognised in Asia in outbreaks, this is the first major outbreak in Australia. The fact that affected litters are seen in multiparity sows indicates this is a recent incursion into these farms. The striking clinical signs naturally caused distress and concern in the staff of affected farms. The onset of winter will reduce the mosquito numbers significantly. The typical seasonal patterns of La Niña results in rainfall changes which can extend mosquito ranges and waterbird breeding patterns. The effects of changes in climatic conditions should not be underestimated.
The presence of large number of wild pigs in northern Australia combined with mosquito movement may have assisted the spread of the virus into the south of Australia
The tropical conditions in the north of Queensland and the large number of feral pigs is going to maintain the Japanese encephalitis virus infection in waterfowl and mosquitoes. As weather patterns change with both season and climatic effects, the virus is likely to periodically move into the southern states. The disease pattern will follow mosquito spread along rivers and wetlands. These areas are popular for water birds, feral pigs, mosquitoes and people who are bitten.
Viruses, resembling Flaviviridae and mosquito-borne diseases are not uncommon and cyclically affect various ranges of species. In Australia 2022 it is pigs with Japanese encephalitis virus. It has been humans with Zika (Centers for Disease Control and Prevention, 2022) and birds with West Nile virus (Nash et al, 1999). There are many viruses in this family and these sorts of occurrences in different species are likely to occur in the future and generally will occur with little warning.
Conclusions
An outbreak of catastrophic rise in stillborn and mummified piglets at birth occurred over a 10 week period in up to 60% of the batch of farrowing sows. Piglets were born with severe congenital deformities particularly affecting the limb development, with limb extension and arthrogryposis and some swelling of the legs. There were other skeletal changes with brachygraphica. Neurological changes were also apparent in some cases with a total loss of brain neural tissues.
In a few cases of piglets that were born alive, congenital tremor could be seen. In other litters, gestation was extended to over 120 days and these sows, at parturition had entire dead litters. There were no obvious clinical signs in piglets born alive, nursery or finishing pigs or in the sows. However, other clinicians did experience boars with seminal changes and rarely a chronic epididymitis. The clinical problem lasted about 10 weeks from the first suspected case following a typical normal pattern as shown in Figure 2.
The Japanese encephalitis virus is transmitted by mosquitoes and the current La Niña occurring in Australia and large rainfalls and migration of water birds are believed to be a major part of the epidemiology. At the beginning of autumn the cases have reduced and stopped. Possible vaccination is being planned to protect the farm against future summer events.
KEY POINTS
- Japanese encephalitis virus (JEV) is a mosquito borne pathogen
- Pig to pig transmission is possible, but it is rare
- JEV clinically is part of the SMEDI complex.
- JEV Genotype 4 can cause catastrophic congenital defects in pigs
- Boars can have a chronic epididymitis
- Viraemia is only 5 days in the pigs and PCR will be negative in the other
- JEV PCR positive results in the dead and congenital deformed piglets.
- Sows will be ELISA positive.
- Antibody analysis can be complicated with cross-reactions between different members of the JEV group of flaviviruses endemic within Australia.


