Many cattle enterprises in the UK employ ‘do it yourself’ artificial insemination (DIY AI) as their main method of getting their cows in calf. DIY AI is a big responsibility which requires a large number of skills and the process should be a key focus when working with farms to achieve optimal fertility. It is essential that techniques should be evaluated not only when there are conception rate problems, and that routine ‘refreshers’ should be offered to all relevant staff at pertinent points in the breeding calendar whether the farm is a block calving or all year round producer. This article looks at the process from tank to cow, highlighting areas in the process that should be addressed.
Conception rate problems in fertility investigations on cattle farms are oft en a multifactorial issue. Poor artificial insemination (AI) technique by individuals performing DIY AI on farm is one possible contributing factor. Conception rate can be affected by poor oocyte quality, metabolic disease, oestrus synchronisation methods, environmental stressors, i.e. heat, semen factors, uterine health and causes of early embryonic death (Figure 1).
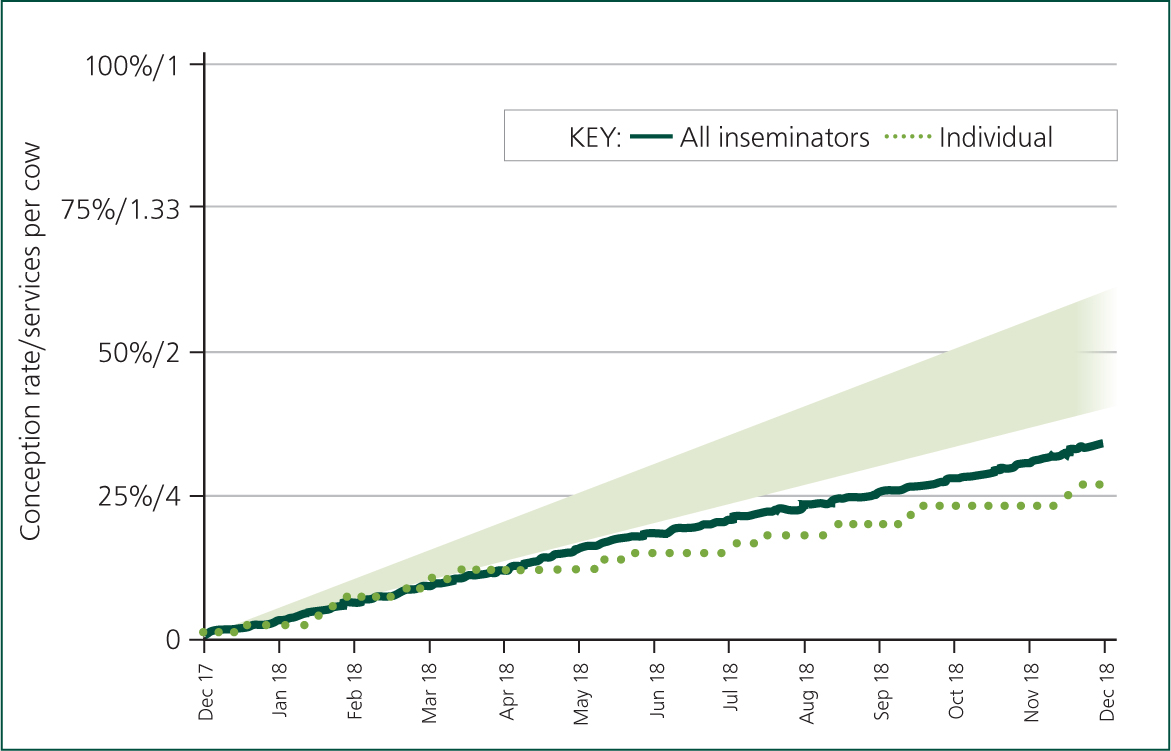
Data analysis can be employed to illustrate whether poor AI technique, especially for specific individuals, is implicated in suboptimal conception rate results (Figure 2). The Australian InCalf study showed that around 45% of DIY AI technicians could increase conception rates by >5% by adopting optimal semen handling and AI practices (Morton et al, 2003).
Once it has been identified that there is a problem with the DIY AI technique on farm, the process needs to be broken down to identify which step, or more commonly steps, is causing the problem. Achieving successful AI and maximising conception rates is a process of marginal gains, as small differences in processes and attention to detail can lead to small, but financially beneficial increases in conception rates. Veterinarians have a role to play in ensuring their clients are achieving optimal results and reaping the benefits from doing their own AI.
The stages that should be part of any investigation of DIY AI are:
- Oestrus detection: the holistic approach to investigating AI cannot only focus on putting the semen in the cow. The fundamentals need to be addressed, such as do the farm staff who detect oestrus (whether or not they also do AI) have a great enough understanding of oestrus detection to correctly identify a cow that is eligible for service. In particular can they recognise the primary and secondary signs of oestrus in cattle (Table 1) and understand when a cow should be served based on the signs she is showing (Table 2).
- The AM: PM rule: if a cow is seen in standing heat in the morning she should be served that aft ernoon, and if she is seen in standing heat in the aft ernoon she should be served the next morning, is a commonly used practice. The aim of the rule is to maximise the chance of conception by ensuring that viable sperm meet a viable oocyte (Hunter et al, 1984). On average the oocyte is released 24 hours aft er the start of standing heat, and is viable for approximately 12 hours, while sperm are viable for approximately 28 hours aft er insemination, so following the AM:PM rule should result in viable sperm meeting viable oocytes. This was recently confirmed by Sheldon (2015) who used accelerometers to monitor cow activity and found that insemination was most successful 11½ hours aft er the onset of increased activity.
Table 1. Primary vs Secondary signs of oestrus in cattle
| Primary signs of oestrus in cattle |
|---|
| Standing to be mounted |
| Secondary signs of oestrus is cattle |
| Head mounting another cow |
| Mounting or attempting to mount other cows |
| Chin resting on another cow |
| Sniffing or licking vulva |
| Bellowing, butting, restlessness |
| Clear vulval slime/swollen vulva |
| Change in behaviour, i.e. not grazing/position she comes into the parlour |
| Change in yield |
| Off food |
Table 2. Managing heat detection
| Sign | Points score |
|---|---|
| Clear vulval slime | 3 |
| Bellowing, butting and restlessness | 5 |
| Sniffing or licking vulva | 10 |
| Being mounted and walking off | 10 |
| Chin resting on another cow | 15 |
| Mounting (or attempting to mount) other cows | 35 |
| Head mounting another cow | 45 |
| Standing heat | 100 |
| The system is designed to be used cumulatively during a 24-hour period, points for observed activities being added up for each cowWhen cows are observed three times a day for 30-minute periods a threshold of 50 points indicates heat | |
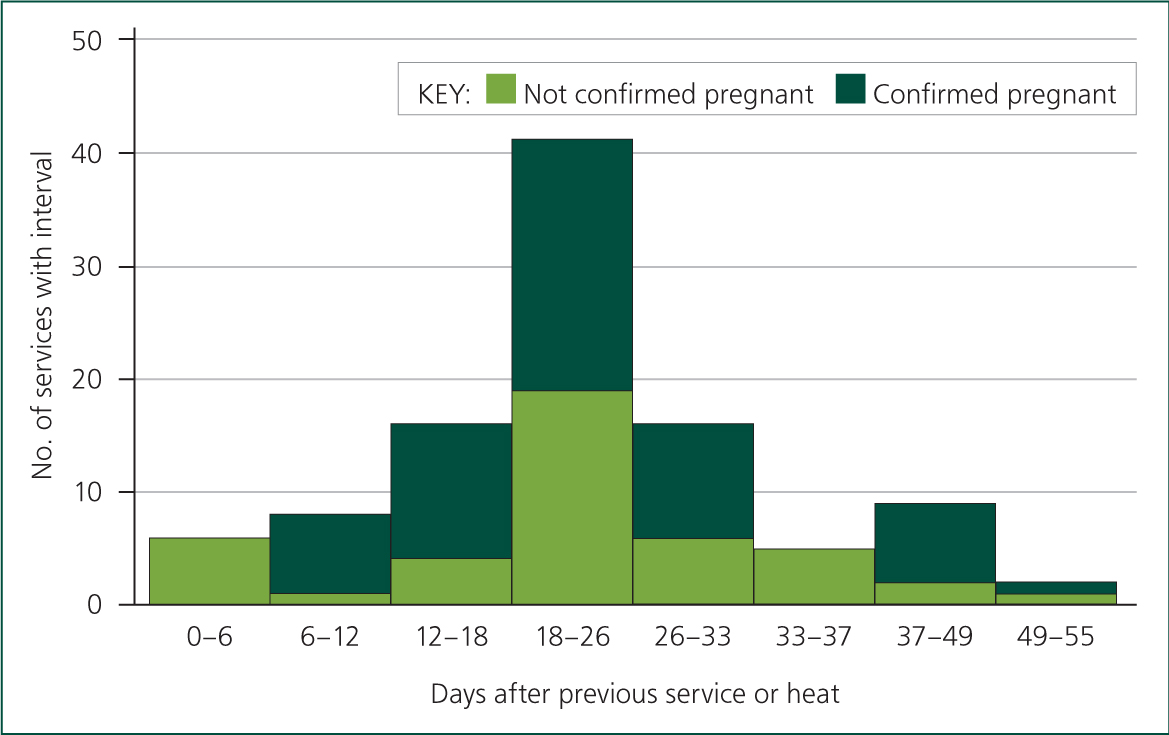
Heat detection accuracy figures can be analysed for the individual (Figure 3) and for the farm team. If they are poor for the entire farm team, the investigation needs to evaluate whether there are factors such as inadequate nutrition, lack of space or slippery surfaces (White, 2006) which are resulting in poor or absent oestrus behaviour, or whether poor detection is due to a lack of time and/or skill across the farm team.
Attention to detail between identification of an animal in heat and insemination is crucial. The bulling cow needs to be safely restrained in a calm environment, preferably with a herd mate. Her records must be checked to ensure that she is eligible for service and to determine which bull she is to be bred to. Gloves, lubricant and paper towels must all be ready and within reach of the cow for the insemination process. Any delays once the semen has thawed puts time pressure on the inseminator, potentially increasing the length of time until semen is placed accurately in the cow, thereby possibly compromising conception rates (Figure 4).

It is recommended that cows should be restrained in a crush or AI stall for the insemination process, for the health and safety of the inseminator and welfare of the cow (Figure 5). This set-up allows a step to be used if necessary, and with the cow safely restrained, the inseminator is under less pressure to perform the insemination quickly since they are in a comfortable and safe position.

- Semen storage: if there is a problem with semen storage on farm then it is likely that the conception rates of all team members will be affected, rather than just an individual. Semen should be stored at a temperature of -196°C in liquid nitrogen in an AI tank. The tank should be kept off the floor (i.e. on a wooden pallet) in a well-lit and ventilated room. The tank should not be moved unless absolutely necessary. If animals need to be served away from the main farm (i.e. a group of heifers at a different holding) a transport flask should be used.
The level of liquid nitrogen in the flask should be measured frequently to ensure it is adequate and that there are no leaks in the AI tank. Many units are on contracts with semen companies where this is done for them, along with liquid nitrogen top ups and the delivery of semen straws.
Semen handling
When investigating an individual's on-farm conception rate, semen handling is a crucial area to focus on. The number of viable sperm in each straw can be affected by an individual's skills, knowledge and attention to detail. It must be noted that poor handling of semen by others prior to the straw's removal from the tank may also affect semen quality. The quality of the semen in the tank can be checked by analysing a prepared straw on a heated microscope stage aft er correct removal and thawing (Chenoweth, 2002).
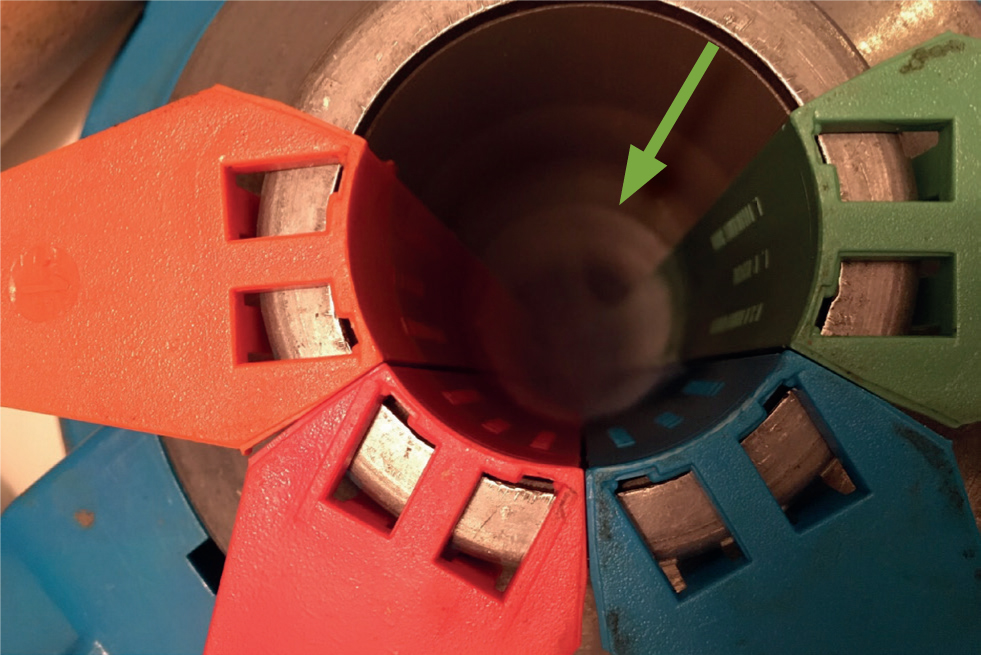
Straws need to be kept at -196°C and not exposed to thawing while in the tank; i.e. the canister should not be brought above the frost line in the neck of the tank (Figure 6). This means that semen in each canister needs to be clearly labelled so that straws do not need to be removed from the flask for identification. Additionally straws should not be packed too tightly into the canisters so that they can be easily removed with forceps. If a canister is brought to the frost line for more than 10 seconds and the individual is unsuccessful at removing the straw, the canister should be placed back into the flask for 20–30 seconds before a second attempt is made.
The straw should be removed with a pair of forceps if possible (Figure 7). If the straw has to be handled with fingers then only the crimped end or area over the plug should be handled, not the areas containing sperm. The straw should be defrosted quickly with the aim of getting the straw from -196°C to +35°C in a water bath as quickly as possible. This is the quick thaw method. This decreases the harmful effects of water recrystallization and rehydration, thus preventing damage to sperm membranes and cytoplasm. The critical temperature for ice crystals to form is between -50°C and 0°C. Rapid progression through this temperature zone means that the semen switches from glassy to a liquid state and ice crystals have insufficient time to form (Diskin, 2018). Three seconds should be the maximum time for moving the straw from tank to tank or tank to thaw flask (Seidel, 2011). The straw should be waft ed before placing it in the water bath so that excess liquid nitrogen is removed to avoid its contact with the water and risk of explosion. Safety goggles should be worn at all times when handling liquid nitrogen.

Semen thawing
Advice on semen thawing times and temperatures should be sourced from the representative selling the semen, as recommendations can vary. For example, some companies recommend that sexed semen is warmed in a water bath of 36°C as opposed to the conventional recommendation of 35°C for 20 secs for a 0.25 ml straw and 40 secs for a 0.5 ml straw. 0.25 ml straws are common place in the UK and it must be noted that due to their large surface area to volume ratio they are susceptible to rapid temperature fluctuations (Diskin, 2018).
The water bath must be preheated and temperature checked prior to removing the straw from the tank. It is recommended that a temperature controlled water bath is used to ease time pressures preparing for AI and ensure accurate temperatures (Figure 7). It is important that the water is changed weekly to minimise contamination in the water bath, and that the temperature is calibrated and checked once weekly. To do this use a standard (clean) thermometer to check that the water in the water bath is at the temperature required.
A stop watch should be used to time how long the straw is in the water bath. While the straw is thawing it should be ensured that all the necessary equipment, i.e. paper towel, gun, sheath, scissors, is ready to hand.
Handling thawed semen
Most substances are spermatotoxic including water, blood and faecal matter, so it is essential that once the straw is cut these substances do not come into contact with the sperm. Once the straw is removed from the water bath (preferably again with forceps or handled only over the crimped end/plug) the straw should be dried with a paper towel and placed straight away into a gun that has been warmed to body temperature. The gun can be warmed while the straw is in the water bath. Ensure that the gun is not too cold or too hot from vigorous rubbing with a paper towel by checking its temperature against the skin on your forearm. Automatic gun warmers can be purchased to remove the variable of warming of the gun and to maintain a constant temperature until the person is ready to inseminate the cow. If the inseminator does not have an automatic gun warmer the loaded gun should be wrapped in a paper towel and put inside (clean!) overalls to maintain it at body temperature.
It is recommended that only one straw should be thawed at a time to maximise conception rates. If too many straws are put simultaneously into the water bath then the overall temperature may decrease to below 35°C, and the time between thaw and insemination may increase. If multiple straws are thawed at the same time it should be ensured that they are not touching one another within the water bath. The time between a straw leaving the tank and insemination should be no longer than 6 to 8 minutes (Diskin, 2018), and the gun should be maintained at body temperature the whole time.
After removing the straw from the water bath, it should be dried and loaded ‘plug end’ down, and cut perpendicularly under the crimped section. The sheath should then be placed on the gun and fixed with the plastic polo. All disposable equipment such as sheaths must only be used once. While metal guns should be boil washed once weekly, a Chamise Sanitaire or plastic sheath can also be used to protect the sheath while it goes through the vagina to prevent contamination going from the vagina through to the cervix. This is especially useful for animals that have had an intravaginal progesterone device prior to service as these can induce a local vaginitis. This use of a plastic sheath that is removed on manipulation through the cervix reduces bacterial contamination at the end of the AI gun post AI, and increases the number of pregnancies per AI for cows having their second or greater number of services (Bas et al, 2011).
Semen placement
At this stage in the investigation a gun should be loaded with a straw containing an echogenic substance (e.g. Sudocrem, Teva UK Ltd.) and a non-pregnant cull cow sourced. This means that the inseminator can ‘inseminate’ a cow and the position of the ‘semen deposition’ can be evaluated.
The individual should be observed in how they handle the cow, as stress can affect reproduction in cattle (Dobson and Smith, 2000), and also how hygienic they are when inserting the gun. The individual should be assessed on how confident they are at locating the cervix and explaining what they are palpating; they should be able to describe the cartilaginous structure of the cervix (often compared to a turkey neck!), the gristly texture of the cartilaginous rings and the sensation of subsequently ‘popping’ through them.
Understanding bovine reproductive tract anatomy is a crucial part of semen placement success. If it is felt that the individual does not have a sufficient grasp on the anatomy required for the AI process, post-mortem uteri specimens can be sourced so that the anatomy can be visualised before live cows are palpated (Figure 8). This is a good way to observe pitfalls in placement of the gun such as avoiding the sub urethral diverticulum, the length of the vagina, the folds in the vagina, the fornix around the protruding cervix, the cartilaginous rings in the cervix and the size of the uterine body.
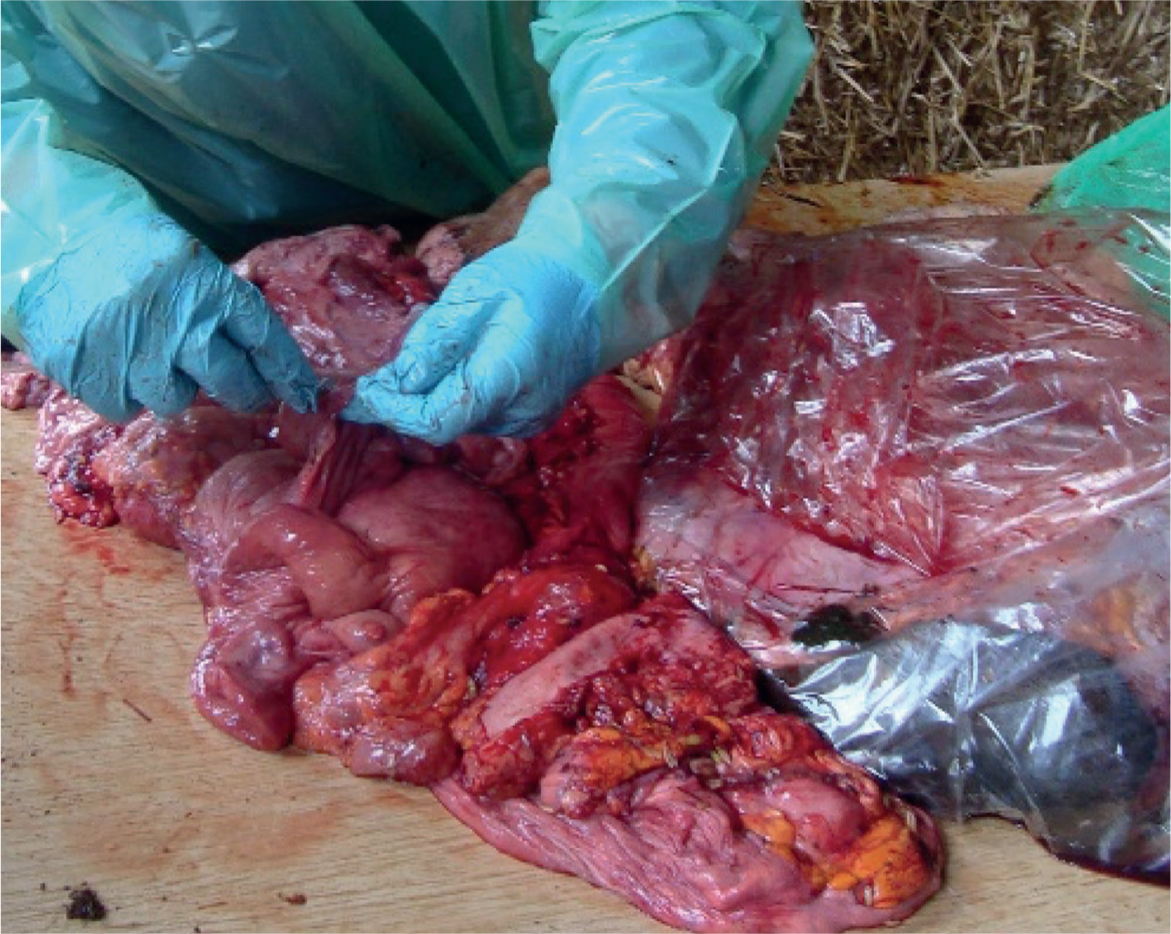
The consensus of several studies is that semen should be deposited in the uterine body, immediately cranial to the cervix (Diskin, 2018). This allows the semen to be deposited evenly between both horns, maximising the chance of conception regardless of which side had the ovulating ovary. The length of the uterine body is usually <2.5 cm (and smaller in heifers), so putting the gun too far into the uterus risks the semen being placed into one of the uterine horns, potentially greatly reducing the chance of conception if the cow ovulated from the contralateral ovary. Putting the gun too far up a horn can also increase the risk of tissue damage to the endometrium with bleeding being detrimental to sperm.
The investigation should also check that the semen is not being deposited too quickly resulting in expulsion of semen up one horn and that the exit of the gun is not blocked by the inseminator's finger. To minimise this risk sheaths are now available that give the gun a rounded tip for minimised trauma and ensure that the semen comes out of the two sides rather than at the end.
To maximise conception rates it is not recommended to split straws. A quick calculation for the cost of a straw vs potentially another 3 weeks open or a fertility intervention does not often add up.
Conclusion
In conclusion, once DIY AI technique has been identified as an area potentially compromising conception rates, there are several steps that should be investigated by the veterinarian with the client on-farm. This process is one that requires attention to detail with small adjustments potentially making a significant improvement to the end result.
KEY POINTS
- Do it yourself artificial insemination (DIY AI) is an area to investigate when investigating conception rates on farm.
- Investigation should focus on oestrus detection, semen storage, semen handling, handling thawed semen and placement of the semen in the cow.
- Attention to detail in all of the steps of DIY AI can improve conception rates by aggregation of marginal gains.
- Understanding of bovine reproductive tract anatomy is a crucial part of accurate semen deposition.


