Haemonchus contortus (often called the barber's pole worm) is a haematophagous trichostrongyle nematode parasite of sheep and goats. Unlike other common trichostrongyle parasites, it mainly causes disease through associated blood loss. Worldwide H. contortus is the most important nematode parasite of small ruminants (Waller and Chandrawathani, 2005). As a result of this importance it has served as the model gastrointestinal nematode (GIN) for the genome project (Laing et al, 2013). It is often described as a ‘tropically adapted’ worm, but this description is misleading as it is a major pathogen in countries with very cold winters, such as Canada (Barrere et al, 2013), Sweden (Lindqvist et al, 2001) and Estonia (Tähepõld, personal observation). In the UK it has been found on 50% of sheep farms (Burgess et al, 2012). Within recent years there have been increasing reports of haemonchosis within southern England, and the combination of anthelmintic resistance and changing climate would seem to make this trend likely to continue (Rose et al, 2016).
The biology of Haemonchus contortus
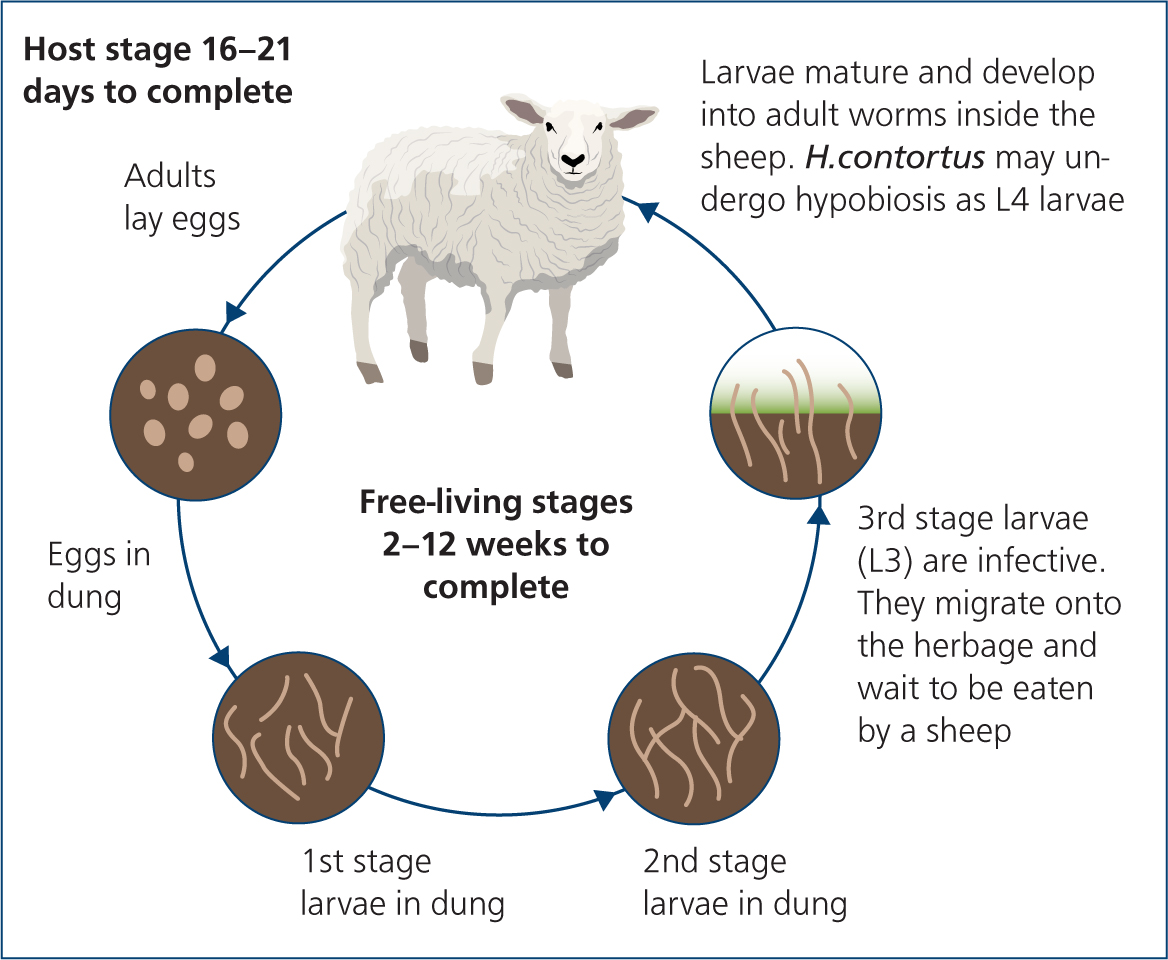
Although the lifecycle of H. contortus is superficially similar to that of many other GIN, it has a significantly different epidemiology to the other GIN species commonly dealt with in UK livestock. Integrated management of this parasite therefore requires an understanding of the factors that influence the survival and development of its life stages (Figure 1).
Adult worms
Adult worms are 2–3 cm long and reside in the abomasum of their host, where they feed on blood obtained using the lancet in their buccal capsule. They have a host preference for sheep and goats, although they can infect cattle (Hogg et al, 2010), deer and camelids, and in sub-tropical regions, the closely related Haemonchus placei is a significant parasite of cattle (Jacquiet et al, 1998; Achi et al, 2003; Jabbar et al, 2014; Taylor et al, 2015). Adult worms have an average lifespan of approximately 50 days, and the female worms are extremely fecund, producing 1300–7000 eggs per worm per day (Getachew et al, 2007; Saccareau et al, 2017), compared with a range of 0–350 eggs per worm per day in Teladorsagia circumcincta (Stear and Bishop, 1999).
From eggs to infective third-stage larvae (L3)
Eggs are passed in faeces and must develop through L1 and L2 stages before reaching the infective L3 stage. This development requires a minimum temperature of 9°C (Crofton, 1965), with optimum development at 25–37°C (O'Connor et al, 2006). Compared with other GIN species in UK livestock, these developmental stages are rapid, taking just 16 days at 10°C, 6.4 days at 20°C and 3.5 days at 30°C (Smith, 1990). This short development phase, combined with the high fecundity of individual worms allows the rapid expansion of populations under optimal conditions, which can lead to the rapid development of clinical disease on farms that have not experienced problems in the past (Figure 2).

As livestock actively avoid areas of pasture contaminated by faeces, infective L3, must translocate away from the faecal pat in order to be ingested. To do this, they require a film of free water to travel in: if long-term humidity has been high, then light rainfall is sufficient to allow this, whereas after periods of drought, heavier rainfall is required (Wang et al, 2014). These results are consistent with the authors' clinical experience of outbreaks in southern England during heat waves or when heavy rainfall has followed warm, dry periods.
Eggs, first stage larvae (L1) and second stage larvae (L2) are vulnerable to temperature fluctuations and to desiccation; however in temperate climates, they are largely shielded from these by the more constant microclimate within the faecal pat (O'Connor et al, 2006). These stages are also very vulnerable to low temperatures, with cool-temperate field studies in Australia suggesting eggs only remain viable for several days after deposition (Barger et al, 1972; Sakwa et al, 2003), therefore long-term survival of eggs, L1 and L2 seem unlikely under UK conditions. L3 are more resistant to variations in environmental conditions; however, they do not tend to survive over winter in Northern Europe, although this may change if winters become milder with climate change (Rose et al, 2015).
As the metabolic rate of L3 is temperature dependent and they have no means of feeding (mouthparts covered by the cuticle), this larval stage also has a shorter lifespan at higher temperatures (O'Connor et al, 2006). In tropical climates, the combination of rapid development and short longevity at high temperatures may be useful for generating clean grazing by rotational grazing (Waller, 1997), and a study in a cool temperate region of Australia found rotational grazing with a rest period of 103 days was effective at controlling H. contortus populations (Colvin et al, 2008). However, rotational grazing patterns commonly seen in the UK (approximately 4-week rest periods) are unlikely to produce significant decay of pasture larval levels.
Early 4th-stage larvae and hypobiosis
After ingestion, L3 moult to become early 4th stage larvae (EL4): these may then either immediately carry on developing through to adult worms, or may arrest their development and undergo hypobiosis until conditions favour further development. This hypobiosis allows the parasite to persist from season to season, despite the limited survival of the free-living stages (Taylor et al, 2015). This feature of the lifecycle is of particular clinical relevance, as it may allow the transmission of H. contortus onto an uninfected holding through the purchase of stock if they are not given quarantine treatments, and it may lead to unexpected clinical disease because of the sudden development of hypobiotic larvae (e.g. at lambing time) (Sargison et al, 2007). This latter disease pattern is commonly seen in countries with cold winters, where on-pasture survival over winter is impossible (Waller et al, 2004). These severe disease outbreaks may occur without noticeable elevations in the faecal worm egg counts the preceding summer and autumn (Tähepõld, personal obvservation). It is also of great significance to the development of anthelmintic resistance, as hypobiotic EL4 may represent the only in refugia population present on a farm during periods of poor L3 survival.
Haemonchosis
Aetiopathogenesis
The disease caused by H. contortus is related to its blood feeding. On average, each worm consumes 0.05 ml per day, but the amount can vary from 0.005 to 0.17 ml (Clark et al, 1962). The worms themselves consume blood, but the blood loss continues after the worm detaches from the feeding site. In addition to the buccal lancet, the feeding is aided by the secretion of calreticulin by the worm, which impairs blood clotting and the immune response to the worm (Suchitra and Joshi, 2005). The majority of clinical signs, therefore, relate to the loss of blood protein and red blood cells.
Other impacts include an increase in the rumen outflow rate, and a decrease in digestion along the gastrointestinal tract as a whole, and loss of amino acids through the action of the worms (Rowe et al, 1988). The developing larvae in the gastric glands cause local distension and the loss of parietal cells (Jackson and Coop, 2007).
Diagnosis
Clinical signs
As the infective larval stages are also haematophagous, the clinical signs can occur before egg-shedding begins. The clinical signs all relate to blood loss, but the time course of the disease process, and thus the clinical signs observed, are influenced by worm burden.
The most consistent clinical sign is pallor, as a result of anaemia. In hyperacute cases (e.g. when animals ingest a very large number of larve in a short period) this is directly related to frank blood loss. In more chronic cases it may reflect loss of red blood cells outstripping the rate of production, or exhaustion of the regenerative capacity. The anaemia and blood loss also results in tachycardia and hyperpnoea and weakness. Heart sounds and pulse quality may reflect the lower viscosity of the blood (Figure 3).
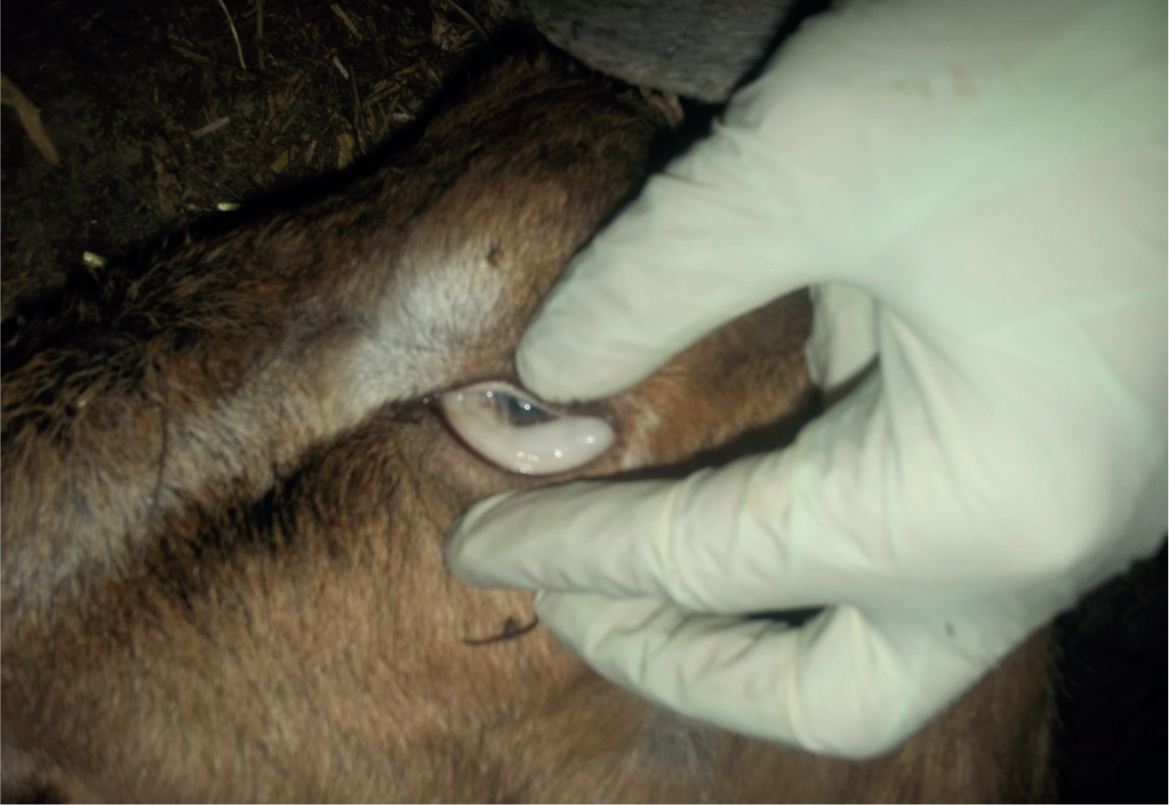
Weight loss, lethargy and poor fleece quality also occur as a result of the blood loss. The loss of blood protein results in submandibular oedema. Diarrhoea is not a feature of haemonchosis; faeces may be scanty and dark.
The packed cell volume (PCV) falls in affected animals, and hypoalbuminaemia is also a feature. Faecal occult blood is also found in haemonchosis, but the usefulness as a diagnostic tool under field conditions is questionable (Rodriguez et al, 2015).
Post-mortem examination
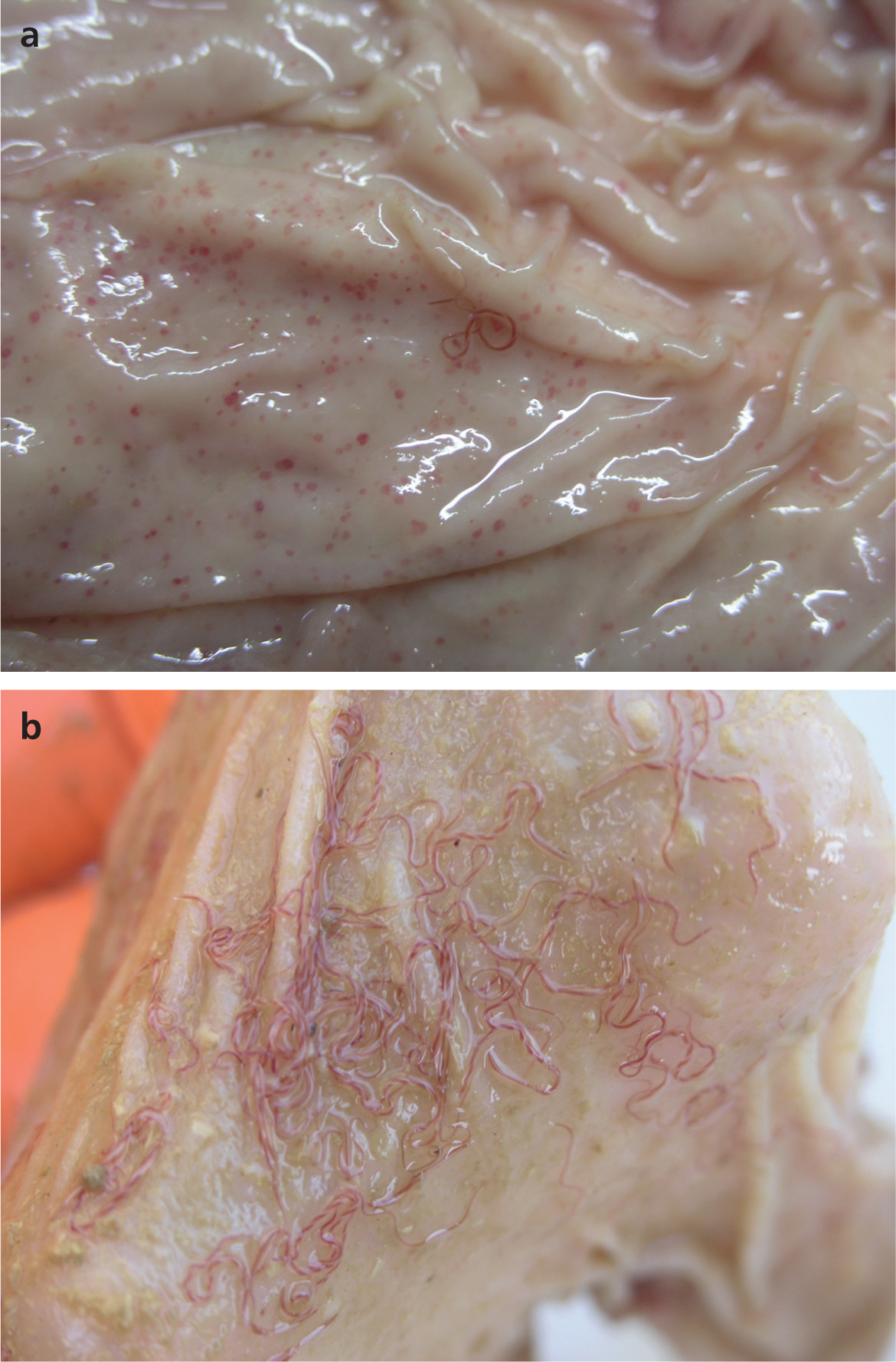
At post-mortem examination, external signs that are indicative of haemonchosis include pallor of the mucous membranes, submandibular oedema and poor body condition. Internally the blood is often watery, and there may be ascites and increased pericardial fluid. There may be pallor of the liver. The abomasal contents are often dark brown and foetid. Adult worms can be detected with the naked eye, with the characteristic ‘barber's pole’ appearance. The abomasal lining may be oedematous. Dark-red petechiae (indicating points of attachment) may be seen, as may nodular changes to the mucosa (Figure 4).
Faecal worm egg count
As the infective larval stages are also haematophagous, the clinical signs can occur before egg-shedding begins. Because of the high fecundity of H. contortus, worm egg counts where this species predominates are often notably high. Counts in the thousands or tens of thousands may be seen where a heavy H. contortus burden is present.
Egg morphology (Christie and Jackson, 1982) and the morphology of L3 larvae (Van Wyk and Mayhew, 2013) can be used to identify H. contortus eggs, but both methods require considerable skill on the part of the operator, and the latter requires significant time to culture the larvae and the resultant relative numbers of the larvae of different species will be affected by differential egg survival and larval hatch.
It has been discovered that peanut agglutinin (PNA) binds to H. contortus eggs but not those of other trichostrongyles. Consequently, fluorescent-labelled PNA can be used as the basis of an assay to determine the percentage of eggs in a sample that are H. contortus (Jurasek et al, 2010). This is a useful, and relatively inexpensive and quick method for determining if, for example, high faecal worm egg counts are due to H. contortus infection. It is currently offered commercially by several laboratories in the UK.
Treatment
H. contortus is susceptible to the available broad-spectrum anthelmintics (benzimidazoles, levamisole, macrocyclic lactones, monepantel and derquantel) (Jackson and Coop, 2007). Benzimidazoles, macrocyclic lactones and monepantel are effective against the hypobiotic larval stages (Stein et al, 2010); levamisole is not (Sargison et al, 2007).
In addition to these broad-spectrum anthelmintics, H. contortus, by virtue of its haematophagy, is also susceptible to other compounds, specifically those that bind to blood proteins. Those available in the UK, in products licensed for use in sheep, are closantel and nitroxynil. As they have a prolonged residence time, because it is protein bound, there is a degree of persistent activity (Hall et al, 1981). As they are dependent on the feeding activity of the worm to be effective, they are not effective against hypobiotic stages. Equally, the therapeutic indices of these compounds are narrow and accurate dosing to bodyweight is required to avoid toxicity.
When considering the need for treatment, as well as choice of anthelmintic compound, the occurence of anthelmintic resistance should be considered (see section below).
Prevention and control
The majority of H. contortus control is similar to that of the control of parasitic gastrointestinal nematodes in general. All that holds true of pasture management, the correct use of anthelmintic drugs, the impact of good nutrition in enabling sheep to mount an effective immune response to the parasite, the potential anthelmintic properties of certain forages (e.g. chicory, Lespedeza spp.) are as true for H. contortus as they are for parasitic gastroenteritis (PGE)-causing nematodes. Consequently, in this section we shall focus in particular on the areas where control must differ from general control of GINs.
One major area of difference is that unlike the situation which pertains with other GIN species, whereby adult female sheep (outwith the peri-partum) seem to be relatively unaffected, thanks to an effective immune response, adult ewes can be clinically affected and even die from haemonchosis. In the authors' experience, haemonchosis is often first detected on farm as a result of morbidity and mortality among ewes, with the problem presumably being curtailed in the lambs by the worm control strategies already applied to them to control PGE. Consequently, one of the first parts of H. contortus control is to ensure that some form of monitoring for haemonchosis is applied to the whole flock.
Anthelmintic resistance and H. contortus
Although there is limited published evidence for anthelmintic resistant H. contortus in the UK, resistance is widespread globally, with reports of resistance against benzimidazoles, levamisole, macrocyclic lactones (including moxidectin) and monepantel within Europe (reviewed in Rose et al, 2015), plus against abamectin/derquantel and closantel in Australia (Rolfe et al, 1990; Sales and Love, 2016). Resistance is thought to develop rapidly as a result of high genetic diversity, polyandrous mating, the enormous biotic potential of individual worms, and anthelmintic treatments at times of limited pasture in refugia populations (Besier, 2001; Gilleard, 2013). It is therefore vital that flock health plans should consider the risks of the introduction of H. contortus onto uninfected holdings, and all efforts should be made to control this parasite sustainably on endemic farms.
Sustainable control of haemonchosis
As noted above, anthelmintic resistance is as much a problem for the control of haemonchosis as it is for the control of all other GINs. The principles of sustainable control are also the same: diversify control away from the reliance on anthelmintics; reduce the use of anthelmintics as much as possible without compromising production, health or welfare; and ensure that the use of anthelmintics is done in such a way as to avoid the selection of resistant strains of nematodes.
Prevention of the introduction of H. contortus onto holdings where it is not already present will be achieved through suitable quarantine treatments (Sustainable Control of Parasites in Sheep (SCOPS) recommends both monepantel and derquantel/abamectin) on arrival, including witholding animals from pasture for 48 hours after treatment and then turning out onto dirty pasture.
Sustainable control strategies that apply equally to haemonchosis as to PGE include:
- Grazing strategies to reduce exposure of sheep to larvae (e.g. cograzing with cattle, use of clean grazing for lambs)
- Use of faecal worm egg counts (FWECs) to determine the need for treatment
- The preservation of an in refugia population through either leaving a percentage of animals untreated at each treatment, or moving the timing of treatment relative to movement
- Correct dosing technique, using correctly stored product, with well-maintained and calibrated equipment, at the correct dose rate for the weight of the animals being treated
- Targeted selective treatment strategies based on lamb growth rates
- The use of bioactive forages.
Readers are urged to consult the SCOPS website for more details (www.scops.org.uk).
FAMACHA© scoring and targeted selective treatment
The pallor as a result of haemonchosis-induced anaemia has been used as the basis of the FAMACHA© (Faffa Malan Chart) scoring system. Developed in South Africa (Van Wyk and Bath, 2002), the conjunctiva of the lower eyelid is compared to a chart and the depth of colour scored on a 5 point scale (1-red to 5-white). The conjunctiva must be used as other mucous membranes have been found to be less reliable indicators, being affected by pigmentation etc. The correlation between FAMACHA© score and PCV, and between FAMACHA© score and faecal egg count (FEC), has been found to be reliable (Kaplan et al, 2004) (Figure 5).

As there are other causes of anaemia a high FAMACHA© score alone cannot be used to diagnose haemonchosis, however, once haemonchosis has been confirmed, FAMACHA© scoring is an extremely useful tool for the development of sustainable control strategies. By identifying the worst affected animals clinically, it allows treatment of those animals that will benefit most from treatment, while leaving unaffected animals untreated. The majority of animals will require no or only one treatment in a season, with only a minority requiring more than one (Van Wyk and Bath, 2002). Scoring up to every 10 days is recommended in South Africa.
This is known as targeted selective treatment, and reduces selection pressure for resistance by preserving a large in refugia population in untreated animals, while simultaneously avoiding production loss by identifying and treating the affected animals. There is evidence that adopting this approach reduces anthelmintic usage, but with no impact on productivity (Leask et al, 2013). Some studies have found that FAMACHA© scoring over-predicts animals affected by haemonchosis (Kaplan et al, 2004), whereas others found that it lacks sensitivity and emphasised the importance of proper training of scorers and regular scoring (Di Loria et al, 2009; Reynecke et al, 2011), but it still reduces anthelmintic usage relative to blanket treatment approaches.
The authors' current practice, in the UK, is to recommend FAMACHA© scoring of adult sheep on farms with haemonchosis problems every 4 weeks throughout the summer months (May–October) with treatment of animals with scores 4 and 5 with a narrow-spectrum product to target H. contortus specifically e.g. closantel or nitroxynil. (Lamb treatments are determined by FWECs or growth rates, and a broad-spectrum anthelmintic is usually recommended, unless clinical haemonchosis seen in lambs, because of the likely contribution of other pathogenic trichostrongyles to lamb FWECs).
During outbreak situations, e.g. when deaths are reported, then treatment of animals with a score 3 is also recommended. Feedback from clients to one of the authors (Crilly, personal observation) is that in the first FAMACHA© scoring session (usually instigated due to the diagnosis of haemonchosis at post-mortem examination) up to 60% of sheep require treatment (i.e. are score 3, 4 or 5), but when FAMACHA© scoring is done routinely less than 20% of ewes require treatment at each scoring session (i.e. are scores 4 or 5).
As most of the research on the use of FAMACHA© scoring has been done elsewhere, assessment of the validity of FAMACHA© scoring under UK conditions and with UK sheep breeds would help place this potentially very useful tool in its proper context.
Barbervax
H. contortus is the target of the first nematode vaccine for sheep. The vaccine was developed at the Moredun Research Institute (Fitzpatrick, 2013) and is currently marketed in Australia (as Barbervax; Wormvax, DPIRD, Albany, Western Australia) and South Africa (as Wirevax; Wormvax, DPIRD, Albany, Western Australia). It uses native antigen from the gut of the worm. Due to this being a hidden antigen (not normally exposed to the sheep's immune system) there is no natural boosting of the immune response, so relatively frequent administration of booster doses is required. The recommended initial course is three 1 ml doses subcutaneously, followed by booster doses every 6 weeks during the risk period. In subsequent years a single injection before the risk period is required, followed by 6 weekly boosters (www.barbervax.com).
A sterile immunity does not result, infection is not completely prevented, but the worm burden is reduced and worm egg output is suppressed (Teixeira et al, 2019). Vaccinated lambs shed 80% fewer eggs than unvaccinated lambs in one study (Bassetto et al, 2018); ewe egg shedding was reduced by 87% and worm burden by 79%, with the peri-parturient rise also suppressed (Teixeira et al, 2019). This effect leads to lower levels of pasture contamination and so reduced challenge. As the efficacy is dependent on the immune response of the sheep, nutrition plays a key role. Good nutrition of sheep is required to get maximum benefit from the vaccine (Bassetto et al, 2018).
The vaccine is not currently available in the UK but may be imported under a special import certificate.
Breeding haemonchosis-resistant sheep
In tropical and sub-tropical areas where H. contortus is the major nematode pathogen of sheep, native breeds show greater resistance to infection, both in terms of reduced worm egg output, decreased impact of infection on PCV, and better maintenance of production compared with ‘improved/commercial’ breeds (Mugambi et al, 1997; Aumont et al, 2003; Amarante et al, 2004; Alba-Hurtado et al, 2010). This appears to be because of a greater and more rapidly activated immune response to the worms, with higher levels of mucosal mast cells, eosinophils and leukocytes in the abomasal mucosa of ‘resistant’ compared with ‘susceptible’ breed lambs; more rapid tissue repair may play a role in some breeds (Bricarello et al, 2004; Shakya et al, 2009; Guo et al, 2016).
It should be possible to breed for more resistant sheep, both through conventional methods such as crossing with more resistant breeds (Amarante et al, 1999; Li et al, 2001), selection of stock for breeding on the basis of phenotypic measures such as FEC (Woolaston, 1992), weight gain in the face of challenge or PCV/FAMACHA© score (Burke and Miller, 2008; Riley and Van Wyk, 2009; Pereira et al, 2016) or, more recently, through the identification of genetic markers of resistance to haemonchosis and the use of these for the selection of breeding animals (Estrada-Reyes et al, 2019; Haehling et al, 2020).
Copper oxide wire particles
Copper oxide wire particles (COWP) are often used as a method of copper supplementation, through administration orally, contained in a capsule. They have been found to reduce the worm burden of sheep infected with H. contortus (Soli et al, 2010; Schweizer et al, 2016). The impact is greatest when the COWP are administered after infection is established (Waller et al, 2004; GalindoBarboza et al, 2011). Other forms of copper supplementation do not have the same effect.
One of the concerns with use of COWP in control of haemonchosis is the risk of copper toxicity. Administration of even small amounts (1 g and 0.5 g) of COWP were found to still have an impact on FWEC (Burke and Miller, 2006); but caution is still advisable.
Forecasting of pasture larval contamination
As discussed above, a combined understanding of grazing history, local weather conditions and parasite epidemiology can be useful for judging how quickly free-living GIN larvae will both develop and persist on pasture, and therefore contribute to the general risk assessing of pasture on farms. However, there are many complex factors that affect pasture and faecal micro-climates, therefore it is not possible to make accurate, specific risk assessments without sophisticated modelling (Wang et al, 2018). Such modelling has been used to predict changes in H. contortus distribution across Europe (Rose et al, 2016), and has recently been expanded to include other parasitic species (Vineer et al, 2020). It would be of great value if this could be adapted to provide regional forecasting for H. contortus, as is already performed for Nematodirus battus (www.scops.org.uk/forecasts/nematodirus-forecast). In addition, there is enormous potential to combine this modelling with the increasing wealth of on-farm data (e.g. pasture mass measurements, individual animal bodyweights, GPS/drone tracking of livestock) with satellite monitoring of grasslands (Ali et al, 2016) to generate truly integrated approaches to sustainable parasitology, land management and food production.
How molecular techniques may aid in haemonchosis control
The recent application of deep amplicon sequencing to elucidate the species composition of gastrointestinal nematode populations (the ‘nemabiome’), has enabled researchers to quantify the relative proportions of different parasite species at much higher throughput than using traditional parasitological techniques (Avramenko et al, 2015). These techniques were recently applied to ovine faecal samples from across Great Britain and revealed the relative abundance of H. contortus to be very low on the majority of farms, but to be greater than 50%, a small number of farms. This study also highlighted regional variation in H. contortus abundance, with a greater number of farms in England having high levels, than in Wales or Scotland (Redman et al, 2019).
Deep amplicon sequencing methods have also been applied to quantify single nucleotide polymorphisms (SNPs) associated with benzimidazole resistance in H. contortus and other nematode species (Avramenko et al, 2019; Sargison et al, 2019). Significant further work has also narrowed down the regions of the H. contortus genome associated with resistance to macrocyclic lactones, levamisole and monepantel (dos Santos et al, 2019; Doyle et al, 2019; Niciura et al, 2019). These technologies offer great promise for further research into the genetics of anthelmintic resistance and field studies to monitor its emergence and spread, as has already been performed for benzimidazole resistance in Haemonchus spp. in Pakistan (Ali et al, 2019).
Further work has also been performed to develop loop-mediated isothermal amplification (LAMP) assays that are able to identify the presence of Haemonchus spp. eggs (Melville et al, 2014) and the presence of benzimidazole resistance SNPs in H. contortus (Tuersong et al, 2020). These techniques are particularly promising for practitioners as they are performed at ambient temperature with minimal equipment, and may be transferred to lateral flow devices for point of care testing, as has been described for the diagnosis of resistance mutations in malaria (Yongkiettrakul et al, 2017).
Conclusion
Haemonchosis has become a more important problem in the UK over recent years and given likely climatic changes and the spread of anthelmintic resistance this trend is likely to continue. As a result of the differences in the epidemiology of H. contortus and in the pathobiology of haemonchosis compared with PGE, it requires a separate approach to other pathogenic GINs of sheep. Fortunately, there are some unique tools available for the control of haemonchosis, and given its worldwide importance as a parasite of small ruminants, further research into haemonchosis is likely to be relatively extensive.
KEY POINTS
- Haemonchus contortus is a globally important trichostrongyle nematode of sheep and goats, and climate change may lead to it becoming a more important parasite in the UK.
- The lifecycle of H. contortus is similar to other gastrointestinal nematodes (GINs), with the exception of overwintering hypobiosis of L4 larvae; it is the high fecundity of the female and the different temperature preferences of this species that produces epidemiological differences.
- Disease is due to blood loss caused by the haematophagy of this parasite; the disease course ranges from hyperacute to chronic.
- Mechanisms for the treatment and control of parasitic gastroenteritis (PGE) will also be effective in controlling haemonchosis, but the haematophagy means that other treatment options are also possible, e.g. closantel and nitroxynil.
- Important developments in H. contortus control include the FAMACHA scoring system and the Barbervax vaccine; further advances in forecasting and molecular techniques for the identification of species and resistant strains of nematodes may further aid in H. contortus control.


