Camelid eyes are large relative to their head and body size. The axial length of the globe is approximately 38 mm. The cornea is ovoid with a prominent curvature, a horizontal diameter of about 25–30 mm, a vertical diameter of 18–22 mm and a thickness of 0.5–0.6 mm (Gionfriddo, 1994; Andrew et al, 2002). The epithelium is relatively thick, made up of 12–15 layers comprising 30% of corneal thickness. The relatively thick epithelium compared with other species is thought to be protective against UV light, dehydration and trauma. The limbus is often defined by a wide heavily pigmented band. The periocular vibrissae and cilia are long and prominent. The eyelid margins are pigmented and do not contain meibomian glands (Gionfriddo, 1993) (Figures 1 and 2).


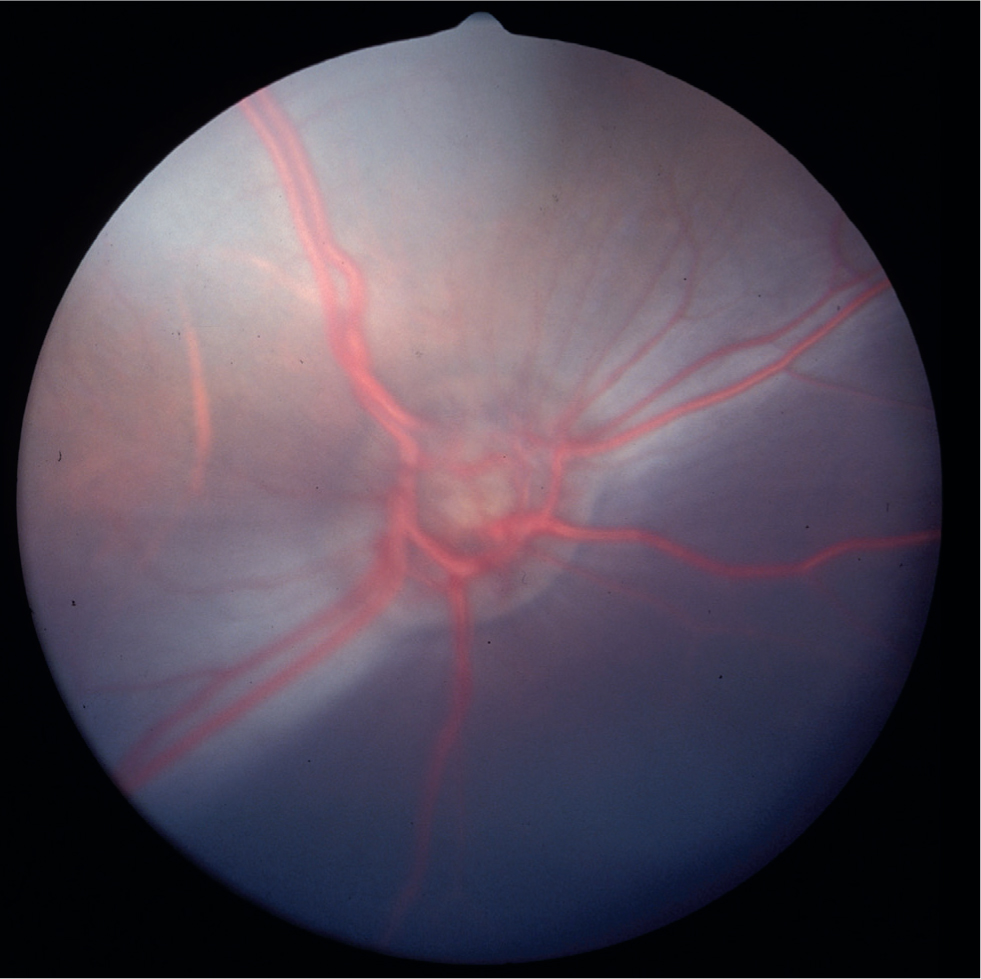
A large haired caruncle of third eyelid contains sebaceous glands that likely contribute to the pre-ocular tear film (Figure 2). The nasolacrimal anatomy is similar to sheep and horses. The iris of camelids is unique with large pleated folds of posterior pigmented epithelium located dorsally and ventrally around the oval pupil rather than true corpora nigra (Figures 1 and 2). Pigmentation of the iris and fundus varies with coat colour where more heavily pigmented coat colours are associated with brown irides and a darker brown fundus colour. The fundus of South American camelids is characterised by a lack of a tapetum lucidum, a variably pigmented oval-shaped optic nerve head, a variably pigmented retinal pigmented epithelium that gives a dark appearance to the fundus (except in colour dilute animals), large prominent retinal blood vessels that lie superficially on the retinal surface, and commonly, a long, white hyaloid artery remnant (Bergmeister's papilla) visible extending from the center of the optic nerve (Figure 3). Vision in alpacas is nearly emmetropic (normal vision) while llamas, especially females, have been found to be slightly myopic (near-sighted) (Willis et al, 2000a).
Ophthalmic examination
Chemical sedation for ophthalmic examination is rarely necessary. Assessment of vision is similar to that of other domestic species. The menace response is elicited by advancing a closed fist quickly toward the eye without creating an air current and observing a blink, globe retraction and/or head retraction. The menace response is learned and is therefore not present in newborn crias. Observation of how the animal navigates in its environment is also helpful. The dazzle reflex (perception of bright light indicated by a blink response) and pupillary light reflexes should be evaluated to assess retinal and optic nerve function. Since a minimal amount of functional retina is required to retain a dazzle or pupillary light reflex, a lack of response is a poor prognostic indictor for vision.
The mean Schirmer tear test value has been reported to be 17–19 mm/minute in llamas (Gionfriddo, 1993, 1994; Trbolova et al, 2012), and approximately 21 mm/min in alpacas (McDonald et al, 2017a) similar to other domestic species. Topical application of fluorescein dye is useful to rule out or assess the extent of a corneal ulcer as well as to assess the patency of the nasolacrimal system. After application, appearance of the dye at the nostril rules out nasolacrimal obstruction. Failure of the dye to appear at the nostril within 5 minutes, however, does not confirm obstruction. Nasolacrimal cannulation and irrigation must subsequently be performed.
Corneal culture and susceptibility are indicated for any corneal ulcer that is slow to heal, progressive and/or refractory to appropriate medical therapy and/or has other clinical features indicating corneal infection such as corneal cellular infiltrate or hypopyon. A pre-moistened swab should be used and ideally the culture collected before applying topical anaesthetic to ensure the most reliable yield. A cytology specimen may be atraumatically collected with a Microbrush® dental applicator and stained with Diff-Quik to evaluate for evidence and type of inflammation and/or infection. The normal ocular flora is similar to other domestic species, where Gram-positive bacterial species such as Staphylococcus spp. and Streptococcus spp. are most common, while presence of some ‘gram-negative species such as Pseudomonas spp. and fungal species such as Aspergillus spp. is normal (Gionfriddo, 1991, 1992).
The mean normal intraocular pressure (IOP) of 12–15 mmHg has been established for llamas and alpacas using the applanation and rebound tonometers (Willis et al, 2000b; McDonald et al, 2017b).
Pharmacologic pupil dilation is indicated for evaluation of the lens, vitreous and fundus. One to two drops of tropicamide may be applied topically for complete pupil dilation within 15–30 minutes that lasts for 4–6 hours.
Anterior segment examination requires a bright light source such as a Finoff transilluminator, ideally with a source of magnification. Fundic examination can be performed using a direct ophthalmoscope which provides approximately 8x magnification. For a wider field of view, a PanOptic retinoscope can be used or indirect ophthalmoscopy can be performed using a transilluminator or flashlight with a 2.2 Pan-retinal or 20 Diopter condensing lens.
Nasolacrimal irrigation may be performed in a normograde or retrograde fashion. The normograde approach requires cannulation of the ocular puncta with a 3.5 French tomcat catheter, then irrigation from ocular puncta to ocular puncta, then after digital compression of the cannulated puncta, irrigation through to the nasal puncta (Gemensky-Metzler, 2008). It is important to be able to perform normograde irrigation since most nasolacrimal anomalies are located distally (e.g. lack of nasal puncta) in camelids (Gionfriddo, 1997; Mangan et al, 2008; Sandmeyer et al, 2011). Retrograde irrigation may be performed using a 5 French 20” urethral catheter through the nasal puncta. The nasal puncta is 2–3 mm in diameter and may be visualised within the nares 15–20 mm proximal to the rostral border of the nasal vestibule (Figure 4). When normal nasolacrimal anatomy and outflow cannot be verified, dacryocystorhinography may be performed. Survey lateral skull radiographs are performed, then the ocular puncta is cannulated and approximately 3–5 ml of a radio-opaque contrast agent is injected into the nasolacrimal duct to identify the site of the obstruction. Alternatively, polypropylene tubing or a 5 French 20” urethral catheter can be advanced from the ocular puncta to the site of the obstruction and is usually palpable beneath the nasal mucosa.

Ocular ultrasonography is indicated when the anterior and/or posterior segment cannot be visualised or for evaluation of orbital disease. A 10–20 MHz probe is preferred. Electroretinography has been performed in camelids presented for blindness to assess retinal function. Waveform amplitudes are higher in camelids than in most normal cats and dogs (Gionfriddo, 2010). Computed tomography (CT) and magnetic resonance imaging (MRI) have been performed in camelids for assessment of intracranial, orbital or sinus disease processes.
Ocular disorders in camelids
A retrospective study found at least one ocular lesion in 6% of llamas presented to veterinary teaching hospitals (Gionfriddo et al, 1997). The prominent nature of the camelid eye predisposes it to traumatic injury (Gelatt et al, 1995). Corneal lacerations and abrasions are the most common ocular lesions seen. Uveitis is also relatively common as a component of systemic infection. Camelids also appear to have a relatively high prevalence of congenital eye lesions.
Diseases of the eyelids and conjunctiva
Blepharitis, inflammation of the eyelids, is fairly uncommon, and is usually concurrent with systemic dermatitis associated with bacterial infection, zinc deficiency, parasites and dermatophytes. Clinical signs include alopecia, scaling, erythema and swelling (Figure 5). Diagnosis is by skin scraping for cytology, culture for dermatophytes and/or skin biopsy.
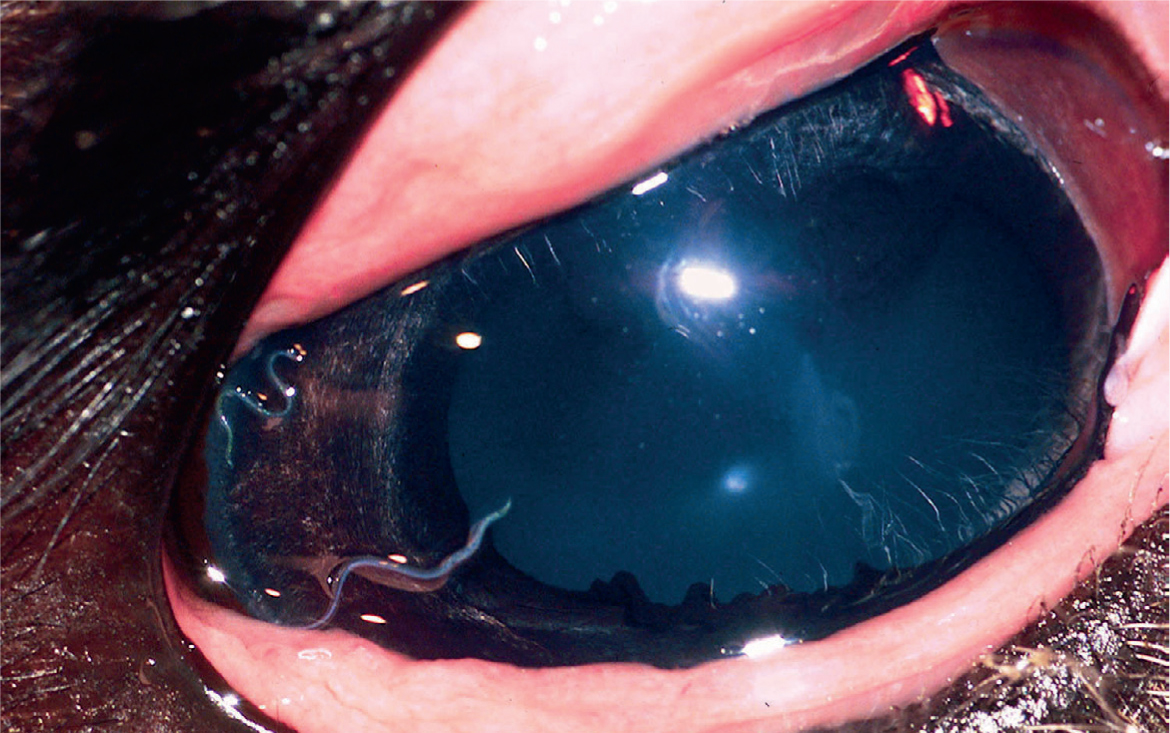
Conjunctivitis involves reddening and thickening of the conjunctiva, with or without corneal involvement. Increased tearing or mucopurulent discharge is often apparent, and may cause crusting or focal hair loss down the side of the face. Bacterial infection, fly irritation, and Thelazia californiensis infestation have been described (Brightman et al, 1981; Gionfriddo, 1994). Culture, cytology, and gross examination of the eye can be used to identify the disorder. T. californiensis adult worms are visible in the conjunctival fornix, and eggs or larvae may be seen on cytologic examination (Figure 6). Diagnosis of fly irritation may be made by exclusion of other causes. Topical antibiotics, protection from flies, and systemic or intraocular antiparasiticals may be helpful. Conjunctival worms may be flushed or extracted manually. Moraxella liquefaciens has been associated with keratoconjunctivitis in a llama (Brightman et al, 1981), but outbreaks of bacterial keratoconjunctivitis have not been reported.
Diseases of the cornea
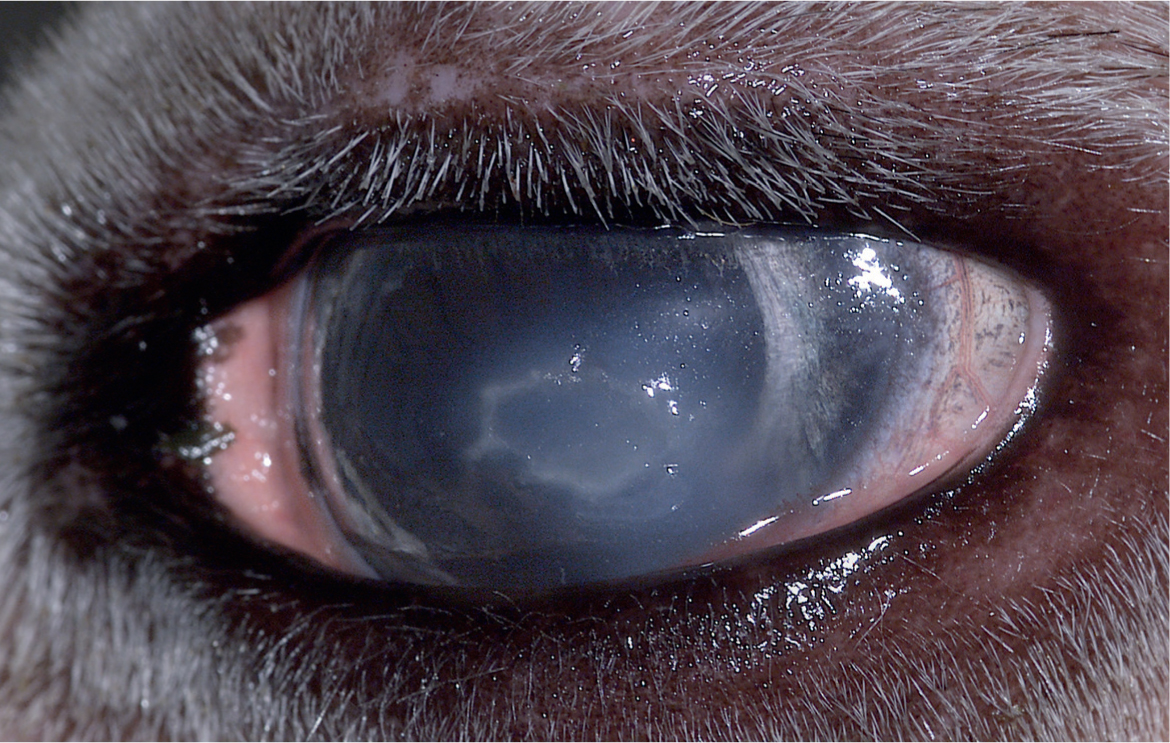
Corneal ulcers are usually caused by trauma and often become secondarily infected (Gionfriddo, 1994; 1997). Primary corneal pathogens are rare, as are diseases affecting multiple camelids. Clinical signs include squinting, light sensitivity, periocular swelling, tearing and focal to diffuse corneal opacity. Corneal oedema is characterised by a bluish ‘ground glass’ appearance and focal oedema indicates a break in the corneal epithelium. Corneal cellular infiltrate indicating inflammation and/or infection appears white to yellow (Figure 7). Fluorescein staining is helpful to identify the location and extent of ulcers. Culture specimens should ideally be collected prior to staining or placement of any substances onto the eye, but proparacaine can be applied if severe blepharospasm impedes sample collection. Cultures should ideally be processed anaerobically as well as aerobically, because up to 20% of samples have yielded anaerobic pathogens (Ledbetter and Scarlett, 2008). Cytology samples should be performed to evaluate for neutrophils, rod or cocci bacteria and fungal hyphae to determine initial therapy pending culture results.
Uncomplicated superficial ulcers usually heal within 5–7 days when treated prophylactically with topical broad-spectrum antibiotics such as bacitracin-neomycin-polymixin B (every (q) 6 hours). Topical atropine (q 12–48 hours) and systemic anti-inflammatory medications such as flunixin meglumine administered early in the treatment course may decrease discomfort and uveitis. If fungi are involved, topical antifungal agents should be added to the treatment plan. Miconazole ointment (q 6 hours) may be used, but it does not adequately penetrate intact corneal epithelium so is not effective against stromal abscesses. Voriconazole penetrates the cornea and has a wide spectrum of efficacy when applied topically q 4–8 hours. Voriconazole may be obtained from a compounding pharmacy or the powdered form for intravenous use can be reconstituted with 19 ml sterile saline to make a 1% solution.
With deeper and/or infected corneal ulcers, more aggressive and longer-term treatment is necessary. Subpalpebral lavage systems may be warranted in camelids requiring frequent treatment, or who become refractory to direct application (Borkowski et al, 2007). Topical antibiotics may be given more frequently (up to 6 times/day) and/or a fluoroquinolone antibiotic such as levofloxacin or ofloxacin may be selected for better corneal penetration (Gemensky-Metzler, 2008). Serum may be collected from the patient to be administered topically (q 2–4 hours) or topical oxytetracycline-polymixin B ointment may be applied (q 6 hours) for anticollagenase effects to slow or stop progression. If the ulcer appears to be worsening, or the depth exceeds 50% of the stroma, the affected cornea may be surgically debrided and a conjunctival pedicle graft applied to provide structural support and blood supply to the defect (Figures 8a,b). The graft is left in place for at least 8–12 weeks but can be trimmed even months to years after the surgery. Perforating ulcers or wounds may be repaired surgically as well (Rodriguez-Alvaro, 2005). Residual corneal scarring may result from chronic or severe ulceration. Corneal tumors have not been reported.
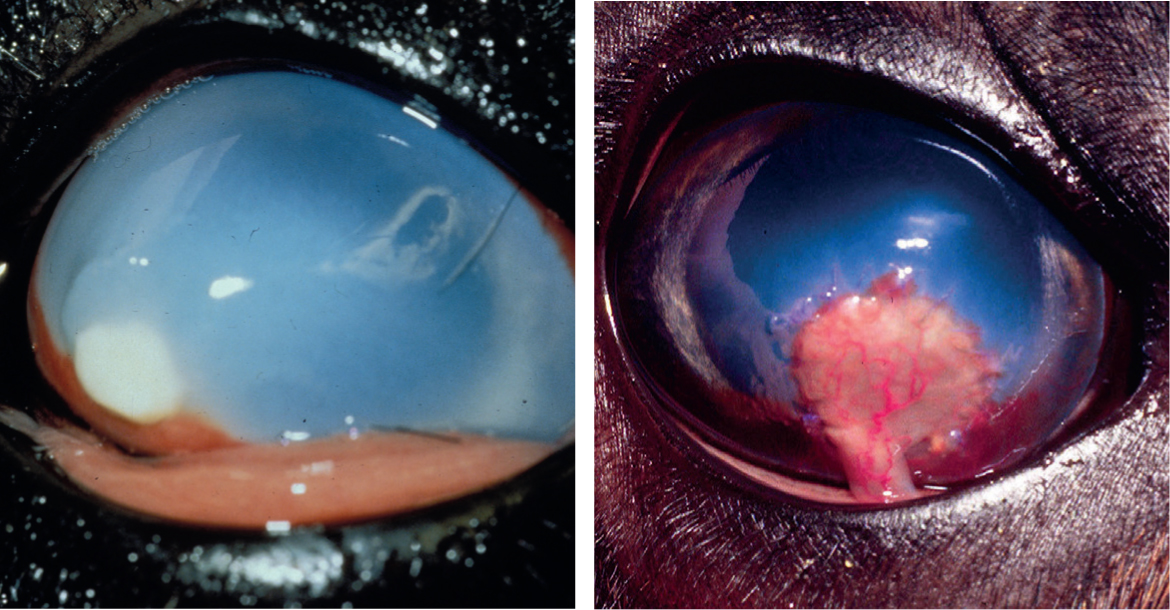
Corneal opacity without superficial ulceration most commonly occurs due to a stromal abscess (Coster et al, 2010), but differential diagnoses should include lipid deposition associated with hypercholesterolaemia (Richter et al, 2006) or corneal fibrosis. Diagnosis via corneal culture or a cytologic specimen is precluded by intact corneal epithelium, but a bacterial or fungal corneal stromal abscess will recruit corneal vessels and elicit ocular pain and inflammation whereas non-infectious infiltrate usually will not. Corneal vascularisation in camelids is typically characterised by thick caliber super-ficial vessels with prominent distal branching (Figure 9).
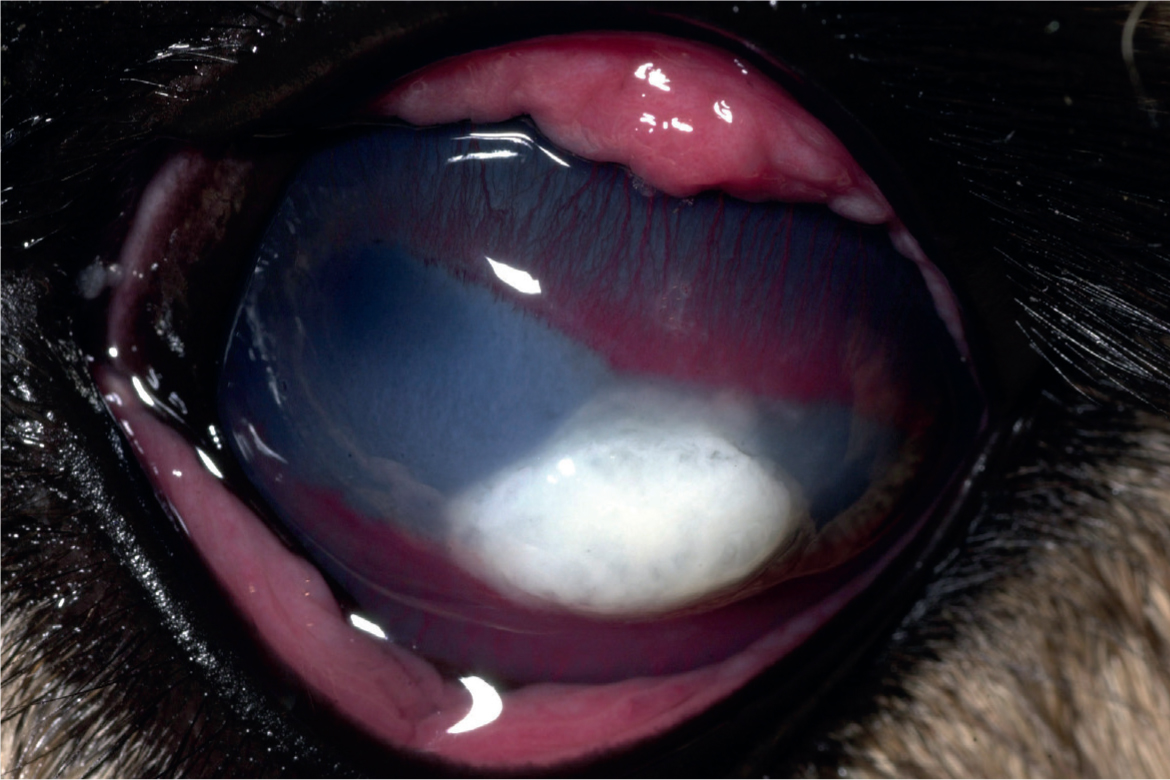
Camelids appear to be prone to developing bullous keratopathy associated with corneal disease, anterior uveitis and cataract surgery (Gionfriddo et al, 1997; Gionfriddo, 2010). Compromise of the endothelial function of pumping fluid from the corneal stroma results in severe oedema, swelling and deformation (‘hydrops’) of the cornea (Figure 10). It is presumed that the endothelium of camelids is more susceptible to dysfunction after insult but the pathogenesis has not been fully elucidated. The density of camelid endothelial cells is similar to that of other species, but it has been suggested that morphologic heterogeneity of the cells may be associated with an abnormal cytoskeleton or stress to the cells (Andrew et al, 2002).

Diseases of the uvea
Anterior uveitis and chorioretinitis may be the result of systemic or local disease, but the specific aetiology is often difficult to discern (Paulsen, 1989; Gionfriddo and Friedman, 1993). Bacterial sepsis, especially in neonates (Adams and Garry, 1992), viral encephalitis (Rebhun et al, 1989), systemic mycosis (Pickett et al, 1985; Richter et al, 2006) and toxoplasmosis have been implicated as specific causes. Protection from light, topical atropine administration once daily, topical and/or systemic corticosteroids or non-steroidal anti-inflammatory agents, and specific antibacterial or antifungal agents may be useful to address the ocular manifestations of these disorders. Anterior or posterior synechiae and cataracts are possible chronic sequelae to iridocyclitis (Figure 11) (Gelatt et al, 1995; Grahn and Cullen, 2001).

Cataracts
Congenital cataracts are commonly diagnosed in camelids, and in most cases, are presumed to be heritable (Gionfriddo, 1994; Gionfriddo et al, 1997; Gionfriddo and Blair, 2002). Small or nuclear cataracts may not obscure vision as the globe and lens grow (Figure 2). However, most congenital cataracts are diffuse and may cause blindness, unless removed (Figure 12). Progressive nuclear and cortical cataracts may partially spontaneously resorb without surgery, but the animal is left with far-sighted vision, and some degree of residual lens opacity and capsular scarring is common (Figure 13) (Gemensky-Metzler, 2008). Progressive cataract resorption often leads to an immune-mediated uveitis recognised by conjunctival hyperaemia, miosis, iris hyperpigmentation and posterior synechia. During resorption, the affected eye should be treated with a topical non-steroidal anti-inflammatory agent such as diclofenac 0.1% or a topical corticosteroid such as 1% prednisolone acetate every 12–48 hours as needed to control the inflammation. Atropine may also be applied topically to prevent formation of posterior synechia.
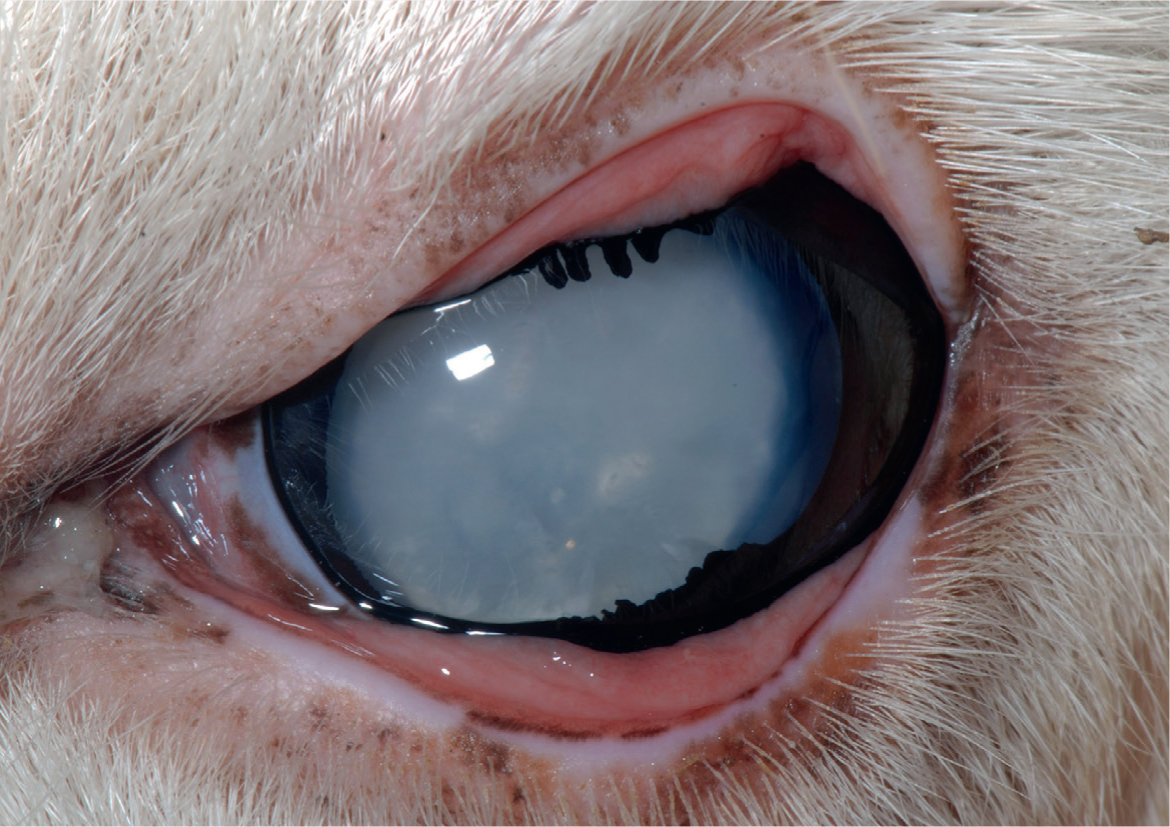
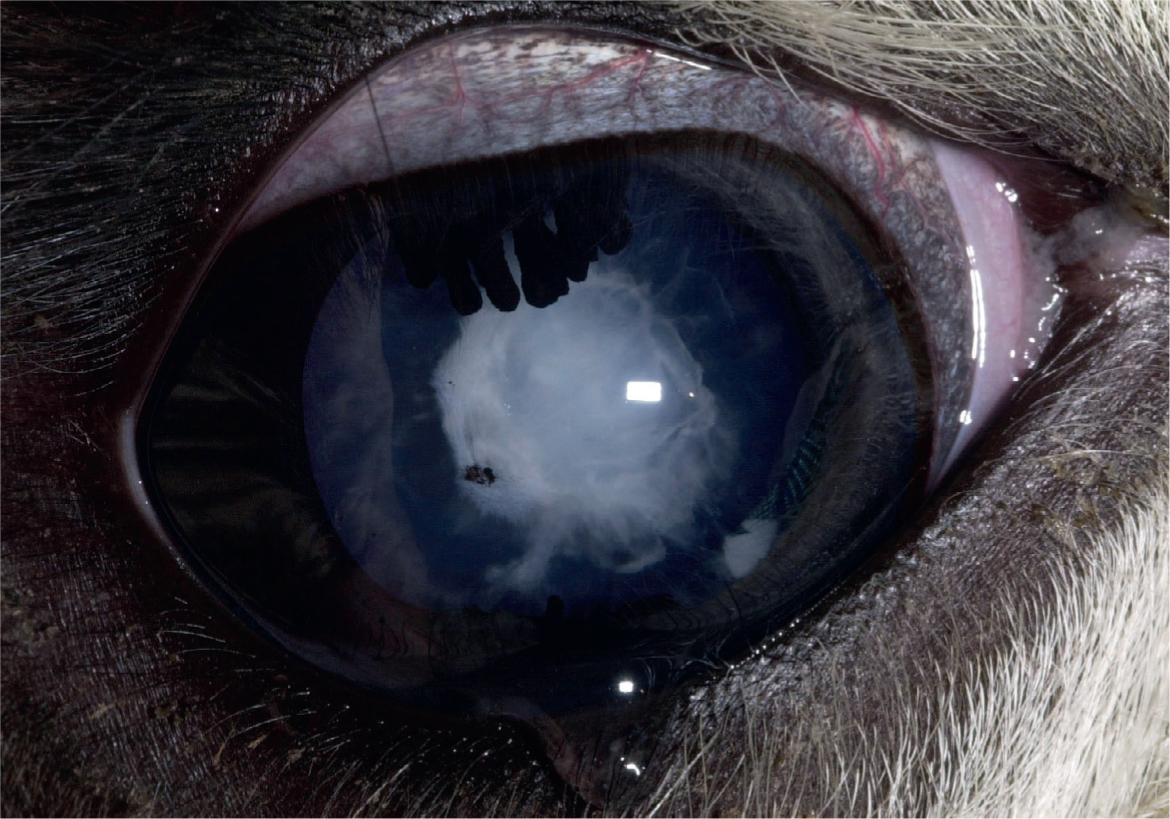
Cataract surgery can be performed in some cases but camelids have an increased risk of corneal endothelial decompensation with subsequent development of severe corneal oedema and bullous keratopathy (Gionfriddo, 1994; Gionfriddo et al, 1997; Gemensky-Metzler, 2008). Use of specific irrigation solutions during the cataract removal procedure may help reduce this risk. Another reported complication of cataract surgery is development of severe secondary glaucoma that required surgical removal of the posterior lens capsule and vitreous to control the intraocular pressure (Powell et al, 2002). Currently, there is no artificial intraocular lens implant available for camelids; the resulting postoperative vision is far-sighted.
Acquired cataracts are uncommon, but may occur in camelids under similar conditions as in other species (Gelatt et al, 1995; Gionfriddo et al, 1997). Previous inflammatory or traumatic lesions, advancing age, and persistent hyperglycaemia are possible risk factors.
Glaucoma and diseases of the posterior segment
Glaucoma has been rarely reported in camelids, but may occur primarily in association with multiple congenital ocular anomalies or may occur secondary to chronic uveitis (Gionfriddo, 1994, 1997), or intraocular surgery (Powell et al, 2002). Topical treatment with intraocular pressure lowering drugs such as 2% dorzolamide or the combination of 2% dorzolamide-0.5% timolol q 8–12 hours may effectively lower the IOP.
Retinal detachment may occur secondary to traumatic globe rupture or can occur subsequent to severe chorioretinitis associated with systemic disease (Gionfriddo, 1994; Gionfriddo et al, 1997). Blindness due to retinal detachment is typically irreversible.
Ocular neoplasia
A variety of intraocular neoplasms have also been described as causes of buphthalmos, uveitis, retinal detachment, epiphora, and blindness. These include lymphoma (Cebra et al, 1995), melanoma (Hill and Hughes, 2009), retinoblastoma (Fugarro et al, 2005), and both teratoid and non-teratoid medulloepithelioma (Hendrix et al, 2000; Schoeniger et al, 2006). Masses may be visible on ophthalmic examination. Removal of the globe is usually curative. Despite the high ultraviolet light exposure sustained in the camelid habitat, squamous cell carcinoma of the globe or eyelids has not been reported.
Congential disorders
Congenital lesions are second only to keratitis in representation among camelid ocular diseases (Gionfriddo et al, 1997). Lesions can range from mild to blinding, and they may or may not be linked to other congenital malformations. Complex skull abnormalities frequently result in microphthalmia (small globes). An-other common congenital anomaly is persistence of the embryonic anterior or posterior perilenticular vasculature (persistent pupillary membranes or persistent hyperplastic primary vitreous/tunica vasculosa lentis (PHPV/PHTVL), respectively) (Gionfrid-do and Blair, 2002). This condition is identified by white or red blood vessel remnants around the pupil margin and/or coursing over the posterior lens capsule. Lens and optic nerve coloboma have also been described (Barrie et al, 1978; Gelatt et al, 1995; Gionfriddo et al, 1997).
Atresia of the nasal puncta of the nasolacrimal duct may be unilateral or bilateral (Gionfriddo et al, 1997; Gionfriddo, 2010; Sandmeyer et al, 2011). The main clinical sign is tear overflow with periocular wetting. Signs are noticed soon after birth and persist, and discharge may become more mucopurulent with chronicity. Secondary conjunctival bacterial overgrowth may occur. Failure of fluorescein to pass from the eye into the nose, inability to flush the nasolacrimal duct, or contrast radiographs may be used diagnostically. Correction involves passing polyethylene tubing from the lacrimal puncta as far as it will go, injection of lidocaine through the tubing to form a bleb in the nostril, incising the mucosa over the bleb, and either pushing the tubing through the submucosal tissue out the incision, or grasping the tip with forceps and pulling it out. Tubing can be sutured to the outside of the nostril and the eyelid and left in place for 2–6 weeks to allow the new orifice to epithelialise. Topical ophthalmic antibiotics, or antibiotic-steroid combinations should be administered 2–4 times daily while the tube is in place. When the tube cannot be palpated or advanced to the expected length of the nasal passage, dacryocystorhinography is helpful to identify the more proximal site of the atresia. In such cases, alternative corrective surgical procedures such as conjunctivorhinostomy may be necessary.
Conclusion
A systematic and thorough ophthalmic examination is critical to identify and categorise ophthalmic lesions. Although much variation in ocular anatomy occurs between species, ocular inflammatory response to injury, infection and other insult are similar. A comprehensive knowledge of the unique ophthalmic anatomy and the common ophthalmic disorders of South American camelids will enable the clinician to accurately interpret ophthalmic lesions, formulate a working diagnosis and develop a treatment plan and prognosis.
KEY POINTS
- South American camelids have unique ophthalmic anatomy and complete ophthalmic examination is integral to accurately diagnosing and treating ophthalmic disorders.
- The most common ophthalmic disorders in South American camelids are corneal disease and congenital anomalies.
- Congenital cataracts that impair vision are common in South American camelids and aphakic vision may be regained after phacoemulsification or resorption of the lens.
- Nasolacrimal atresia is common in South American camelids and can be corrected surgically by creating an opening in the nasal vestibule over the site of termination of the nasolacrimal duct.
- South American camelids are resistant to the development of ocular squamous cell carcinoma.


