Copper poisoning in sheep is seen in both the chronic and acute forms relatively commonly. Acute poisoning is usually from an accidental overdose of copper, although poisoning as a result of gradual accumulation is the cause of chronic copper poisoning. Excess ingestion leads to accumulation of copper in the body tissues most especially the liver. Sheep are particularly susceptible to copper poisoning compared with other ruminant species (McDonald et al, 2011). Some breeds of sheep are particularly susceptible to copper poisoning, such as North Ronaldsey, Texels, Sufflolks and Blue Faced Leicsters, whereas breeds such as Scottish Blackfaces and Swaledales are less susceptible to it. In this author's experience, the most common time copper poisoning is seen is when sheep are fed cake or minerals intended for cattle. Prognosis is grave once the clinical sign of jaundice appears (Scott, 2013).
Clinical signs
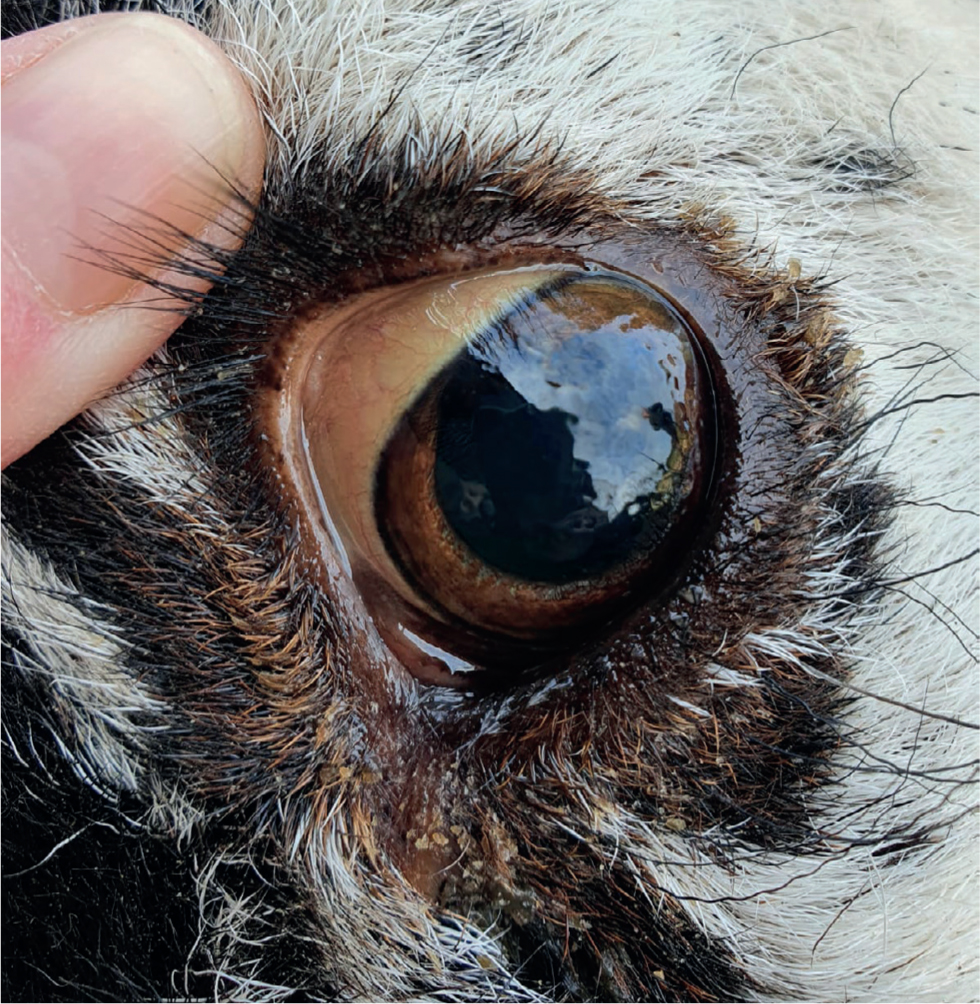
Acute copper poisoning usually leads to sudden death with a very jaundiced carcass with gun metal kidneys and a bronze coloured liver on post-mortem. If the animal survives long enough to develop chronic signs and haemolysis then necrosis of the hepatic cells of the liver, jaundice, loss of appetite are seen and possibly coma leading to death. Some sheep will head press against walls because of the toxin build up in the body. In subacute cases, jaundiced mucous membranes including conjunctiva (Figure 1) can be seen with very dark red (almost black) coloured urine as a result of the haemolysis of red blood cells. Some sheep will pant excessively especially in early cases when anaemia is present.
Pathogenicity
Acute copper poisoning may follow intakes of 20–100 mg of copper/kg in sheep (Blakley, 2013). In chronic cases there can be a gradual accumulation of copper in the liver of sheep until the danger level of around 1000 mg/kg fat free liver dry matter (DM). Poisoning has been recorded in areas where the herbage has contained copper levels of 10–20 mg/kg DM and low levels or copper antagonists such as molybdenum (McDonald et al, 2011). Copper accumulates in the lysosomal cells in the liver and chronic poisoning occurs when the copper storage capacity of these cells becomes exceeded. This causes a sudden release of copper into the peripheral circulation and it is this that leads to haemolysis of red blood cells and death (Sargison, 2008). Some dietary factors may be protective, e.g. high levels of protein, zinc, or iron. Chronic copper toxicosis is more likely to occur with low dietary intake of molybdenum and sulphur as these inhibit copper absorption. Reduced formation of copper molybdate or copper sulphide complexes in tissues impairs the excretion of copper in urine or faeces (Blakley, 2013).
The lysosomal rupture and release of the large concentrations of copper into the general circulation of the sheep occurs when the hepatic concentrations exceed 15000 μmol/kg DM. Subclinical damage starts at a much lower level than this, and it is the subclinical damage that is associated with ill-thrift (Sargison, 2008). The haemolytic crisis may be precipitated by many factors, including transportation, handling, weather conditions, pregnancy, lactation, strenuous exercise, or a deteriorating plane of nutrition. It is particularly exacerbated if concurrent liver disease is present such as chronic liver fluke or ovine white liver disease, as present in cobalt deficiencies. This excess copper that is released causes the intravascular haemolysis demonstrated in the clinical signs associated with the condition as a result of the element's interaction with red blood cell membranes. During this haemolytic crisis, the blood concentrations if sampled can reach copper levels of 38–100 μmol/litre, where the normal range is 9–23 μmol/litre. The debris from these haemolysed red blood cells can lead to blockage of the renal tubules, which leads to renal failure and a resulting accumulation of metabolites that are toxic to other tissues including the brain, which can lead to the head pressing sometimes seen as a clinical sign (Sargison, 2008).
Phytogenous and hematogenous factors influence secondary chronic copper poisoning. Phytogenous chronic poisoning is seen after ingestion of plants, such as subterranean clover (Trifolium subterraneum), that produce a mineral imbalance and result in excessive copper retention. The plants that are not hepatotoxic contain normal amounts of copper and low levels of molybdenum. The ingestion of plants such as Heliotropium europaeum (Europeum heliotrope) or Senecio spp. (Ragwort) for several months may cause hematogenous chronic copper poisoning. These plants contain hepatotoxic alkaloids, which result in retention of excessive copper in the liver. In dogs with liver diseases such as chronic active hepatitis (CAH), the primary clinical signs may resemble those of chronic copper poisoning, which can be attributed to the liver damage and subsequent retention of excessive copper; however, it is not clear whether CAH causes the accumulation of copper in the liver or is the result of accumulation (Blakley, 2013).
Diagnosis
In sub-acute copper poisoning, several days or weeks before the haemolytic crisis, liver enzymes, including alanine aminotrans-ferase (ALT) and aspartate aminotransferase AST (above 105 iu/litre), are usually increased. AST concentration can be elevated for several days to weeks before the haemolytic crisis, with values commonly >400 u/litre, normally 45–134 u/litre (Scott, 2013). Gammaglutamyltransferase (GGT) can be more than 10 times the normal value and serum bilirubin will be markedly increased (Scott, 2013). During the haemolytic crisis, methaemoglobinaemia, haemoglobinaemia, and decreases in packed cell volume (PCV) and blood glutathione are usually seen (Blakley, 2013). Serum copper concentrations at this stage can be used and these are often above the normal range of 9–20 μmol/litre although this can be associated with other inflammatory conditions of the liver, so it is important that there is also a history of overfeeding or over-supplementing copper.
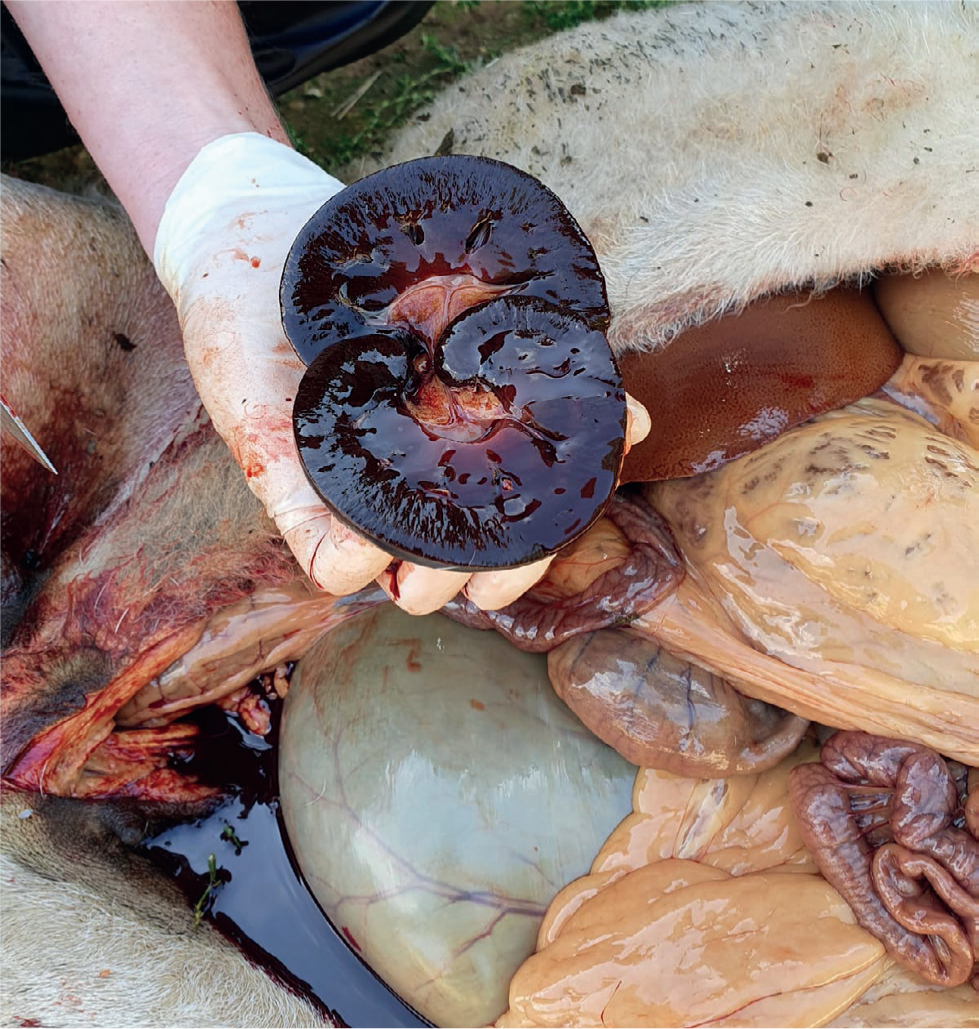
On a blood smear examination, Heinz bodies can often be seen on the red blood cells with basophilic stippling, anisocytosis, and polychromasia (Giandinis et al, 2009). This is due to the strong oxidative damage caused by copper on the red blood cells. Post-mortem examination reveals a jaundiced carcass, a bronze coloured liver (Figure 2) and gun metal coloured kidneys (Figure 3). The carcasses are often dehydrated as well, and urine is dark red or black coloured. If a firm diagnosis is required such as for insurance claims, then the kidney can be sent for copper concentrations, if the level is over 314 μmol/kg DM then death as a result of copper poisoning is confirmed. Liver copper concentrations are often not useful at this point because of the large amount of lysosomal copper release from the liver (Sargison, 2008). There can be other conditions associated with these clinical signs, so it is important to obtain a good clinical history in cases where copper poisoning is suspected.

Treatment and control
Copper poisoning is often a group diagnosis and as such the animals must be treated as a group. Unfortunately, in the early stages blood samples are of limited benefit in detecting which animals need to be treated as the lysosomal damage has not occurred yet. Measuring the AST as above can be useful in some cases and if the level is over 105 iu/litre then this gives a clinical suspicion of the disease. There are some treatments available (as per Table 1); in the past treatment with ammonium tetrathiomolybdate was administered subcutaneously (SC) at a rate of 3.4 mg/kg on 3 alternative days. However, this is banned in food producing animals under EU law. There has also been reported treatments with sodium calcium edetate at a rate of 70 mg/kg. This is diluted to a 5% solution with normal saline and injected intravenously on 2 consecutive days. Supportive therapy with antibiotics, anti-inflammatories and intravenous fluids therapy is highly beneficial for survival as these help with the dehydration and secondary lung infections. If the ration of the sheep has been determined to be high in cooper and low in antagonists, then molybdenum or sulphur can be added either directly into the diet or through water supplementation and occasionally pasture supplementation of 0.25 kg/ha sodium molybdate (Sargison, 2008). Penicillamine (50 mg/kg/day, per os, for 6 days) or calcium versenate may be useful if administered in the early stages of disease to enhance copper excretion. Vitamin C (500 mg/day/sheep, SC) has been shown to reduce oxidative damage to red blood cells during the haemolytic crisis (Blakley, 2013). Minervino et al (2018) found that zinc supplementation at 300 mg/kg DM can be useful in preventing excessive hepatic copper accumulation in sheep exposed to high dietary copper levels. Fresh seaweed offered to sheep with symptoms of copper toxicity appeared to be therapeutic, leading to a reduction in blood plasma copper level (Wiener et al, 1977). It is essential to ensure that the source of copper poisoning is removed to prevent any further accumulation and any more damage to new animals.
Table 1. Treatments for sub-clinical copper poisoning
| Drug | Method of administration | Concentration | Frequency | Advantages/disadvantages |
|---|---|---|---|---|
| Ammonium Tetrathiomolybdate | Sub cutaneous | 3.4 mg/kg | 3 consecutive days | Banned under EU food production laws |
| Sodium calcium edetate | Intravenous in 5% dilution solution | 70 mg/kg | 2 consecutive days | Limited evidence as to efficacy |
In terms of prevention, it is the balance of the availability of copper in the diet with the presence of copper antagonists, such as iron, sulphur and molybdenum, that should be addressed, rather than just the ingestion of copper alone. It is recommended that housed sheep should not receive copper supplementation either just before or after housing because of the lack of antagonists present. If the feed or forage fed to the sheep is high in copper this can potentially lead to copper poisoning so things to avoid are (Sargison, 2008):
- Pasture, silage and root crops grown on ground to which large quantities of pig or poultry manure has been applied
- Distillery by-product feeds such as dark grain produced from copper whiskey stills
- Concentrate feeds containing palm oil or mallassed sugerbeet pulp (wholegrain cereals contain relatively low levels of copper)
- Milk provides highly available source of copper and young animals are very efficient at adsorbing copper so dams should not be fed copper rich diets
- Access to cattle minerals
- Copper sulphate footbath
- Fungicide-treated plants.
Conclusion
Copper poisoning will lead to haemolysis and clinical symptoms such as jaundice and dark coloured urine are observed if the sheep lives for long enough. As these clinical signs can be observed with other diseases, a thorough clinical history must be obtained. If death occurs, the kidneys will be dark gun metal coloured, the carcass will be jaundiced and the liver bronze coloured. There are various treatments but they can be of limited effect. The most important thing is to minimise the animal's (and future animal's) exposure to the copper.
KEY POINTS
- Depending on if copper poisoning is chronic or acute, clinical symptoms vary from jaundice, head pressing and dark coloured urine to death with dark coloured urine.
- Poisoning can occur from over supplementation or feeding, but also in the normal ration from a misbalance in copper and its antagonists.
- Two main treatments are given with varied success — ammonium tetrathiomolybdate and sodium calcium edetate.
- Various control and prevention measures are applicable and these centre around reducing the sheep's exposure to copper.


