As in dairy cattle, intramammary infection in sheep and goats is a major challenge to the health, welfare, productivity and milk quality of dairy small ruminants. The frequency of clinical mastitis is significantly lower than in cattle, but the lack of foremilking in many units means that low-grade mastitis may be overlooked. Clinical mastitis in sheep and goats is often of greater severity than in cattle, with death or culling frequently resulting. Subclinical mastitis occurs in dairy sheep and goats just as it does in cattle, with similar impacts of reduced milk production and reduced milk quality. The detection of subclinical mastitis in small ruminants, especially goats, is complicated by the changes in somatic cell count that occur throughout lactation independent of intramammary infection (Luengo et al, 2004; Koop et al, 2012), and peculiarities of milk secretion that render some automatic counting methods markedly less accurate than in cattle (Dulin et al, 1982).
Mastitis in dairy sheep and goats is predominantly caused by Gram-positive bacteria, in particular Staphylococcus aureus and coagulase-negative staphylococci. Contagious, rather than environmental, transmission predominates.
Given these differences between mastitis in cattle and in sheep and goats, a small ruminant specific approach is required when dealing with dairy sheep and goats, in order to apply the mastitis control tools common to all species correctly.
Prevalence and impact
The annual incidence of clinical mastitis in dairy sheep and goats is estimated to be around 5% (Contreras et al, 2007). This is much lower than the 13–40% incidence of dairy cattle (Jamali et al, 2018), or 50–70 cases per 100 cows per year at the most recent UK study (Bradley et al, 2007). However, death or culling results in up to 90% of cases of clinical mastitis in dairy ewes (Olechnowicz and Jaskowski, 2014).
The annual incidence of subclinical mastitis is much more variable, but the range of 5–30% has been described (Bergonier and Berthelot, 2003; Contreras et al, 2007). Subclinical mastitis is the primary cause of the ‘milk-drop syndrome in ewes’ (i.e. reduced milk yield of lactating ewes, with no clinical signs specific to a disease) (Giadinis et al, 2012). Other financial losses can result from death of clinically-affected animals, early culling of affected ewes and does, veterinary expenses and downgrading of the quality of milk produced, because of changes in its composition (Vasileiou et al, 2019a). Curd yields from milk produced from subclinical mastitis affected udder halves are reduced significantly (Leitner et al, 2004, 2008). This reduction in milk solids is particularly economically significant in small ruminants as the majority of milk is used in cheese and yoghurt production.
S. aureus in milk is a potential zoonotic hazard because of the heat-stable enterotoxins it produces. This risk is particularly high for raw milk cheese manufacture (Van den Brom et al, 2020).
Mastitis is a painful condition, and consequently it is considered one of the most important welfare challenges to the sheep industry (European Food Safety Authority, 2014).
Organisms
In general, bacteria that are capable of causing mastitis in cattle have the potential to cause mastitis in sheep and goats. While Streptococcus uberis is the most frequent cause of mastitis in dairy cattle (Bradley et al, 2007), this species is rarely identified in small ruminants. In these species, the prevalence and importance of Staphylococcus spp. is very different. Staphylococcus spp. are the most common pathogens identified in mastitis cases (Deinhofer et al, 1995; Bergonier et al, 2003; Dore et al, 2016). The most significant species is S. aureus which tends to be associated with more severe clinical symptoms (Bergonier et al, 2003; Koop et al, 2012). One historic study has also shown that Staphylococcus simulans is more likely to cause clinical mastitis (Deinhofer and Pernthaner, 1995), though not typically as severe as S. aureus. The other non-aureus staphylococci are more likely to cause persistent intramammary infection, which may result in elevated somatic cell count and reduced milk yield. Corynebacterium spp. are also worthy of note; aside from Corynebacterium pseudotuberculosis, which causes caseous lymphadenitis (CLA), these bacteria are of limited importance in udder health and are unlikely to cause clinical mastitis or elevated cell counts (Koop et al, 2010). However, they are readily killed by effective post-milking teat disinfection (Enger et al, 2016), and so herds that identify a high prevalence of Corynebacterium spp. should review their parlour routine.
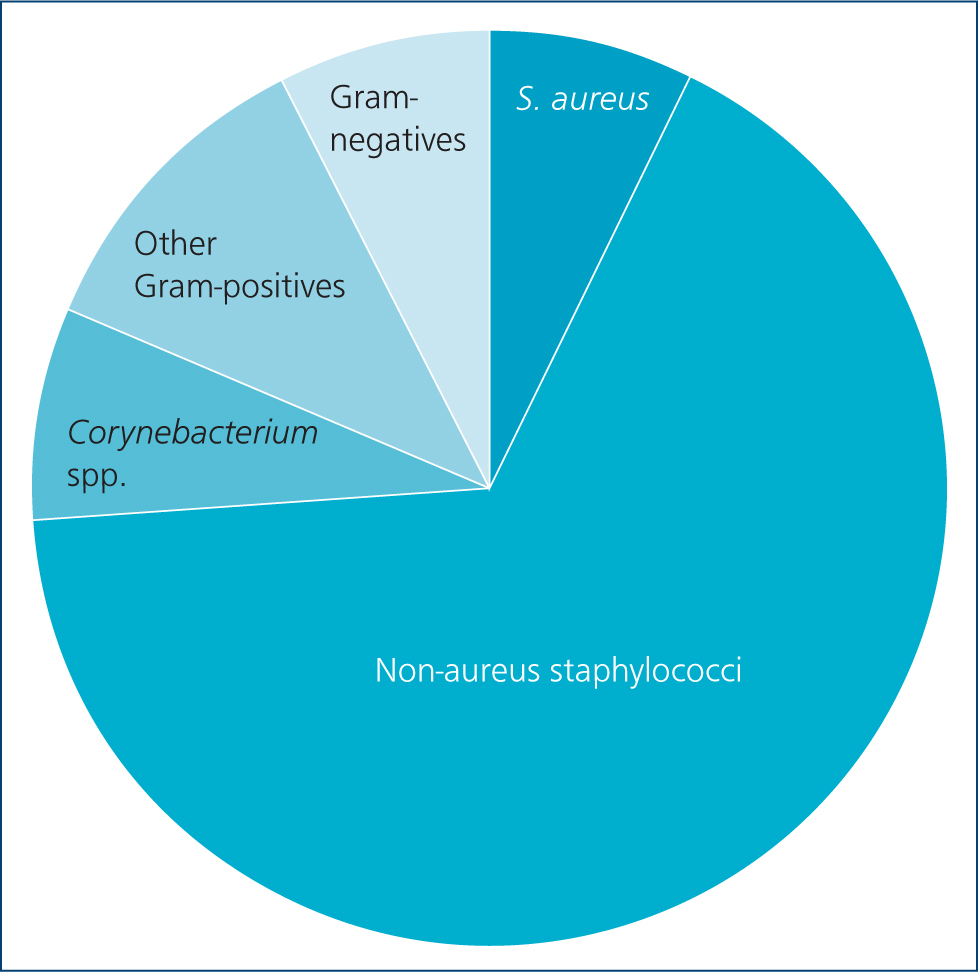
Bacteriological data have been collated from a convenience sample of small ruminant dairy mastitis cases submitted to QMMS over the past 5 years: 63% of samples showed a pure growth, 30% showed mixed growth and 7% showed no significant growth. Figure 1 shows the relative prevalence of different groups of bacteria. Staphylococcus spp. were most frequently isolated, representing 74.3% of bacterial species — similar to the figures reported in other studies. Staphylococcus aureus accounted for 10.1% of Staphylococcus spp. and was present in 7.7% of mastitis cases. Corynebacterium spp. represented 8.1% of isolates, other Grampositives 9.6%, and Gram-negatives only made up 7.8%.
Infection with the lentivirus maedi visna virus can result in an indurative mastitis in ewes. These ewes have an increased somatic cell count but no gross changes to milk (Christodoulopoulos, 2006). Production is reduced. There is variable evidence of a negative effect of maedi visna virus infection, where no udder changes occur, on milk production (Ploumi et al, 2001; Juste et al, 2020). The caprine equivalent, caprine arthritis encephalitis virus, does not seem to produce reductions in milk yield in the early seropositive stage relative to seronegative animals, although fat and protein levels are reduced (Turin et al, 2005; Kaba et al, 2012). Milk yield declines in seropositive animals over time (Smith and Cutlip, 1988; Kaba et al, 2012). Somatic cell counts are increased (Nord and Adnoy, 1997). These viruses are shed in all body fluids; cross-transmission between sheep and goats can occur (Shah et al, 2004). Horizontal transmission is believed to be primarily via the respiratory route; the prolonged housed periods of the majority of dairy small ruminants therefore increases the risk of horizontal transmission. Vertical transmission in milk and colostrum is also important. Serological testing is the main method of detection of infection. There is no vaccine. Control measures include testing and culling, snatch-lambing or kidding, separate rearing of youngstock, and sourcing new animals from accredited free sources (Reina et al, 2009).
Contagious agalactia (Mycoplasma agalactiae) is absent from the UK, and is notifiable. Clinical signs include mastitis, agalactia, abortion, swollen joints, swollen eyes and pyrexia. Further information can be found here: https://www.gov.uk/guidance/contagious-agalactia.
Risk factors
The risk of intramammary infection rises through lactation, and in later parities (Moroni et al, 2005; Koop et al, 2013; Hristov et al, 2016).
Damage to the teats, which compromises the udder's defences, are major risk factors for mastitis. Incorrect milking machine settings and maintenance will damage the teat and increase the transfer of bacteria between teats and so increase the risk of mastitis (see later section), while peri-milking hygiene (e.g. pre-and post-milking dipping) and milking order will also affect transmission rates.
Poor udder conformation or teat placement (Casu et al, 2006; Koop et al, 2013), increases the risk of mastitis — this is presumed to be because of the increased likelihood of suckling or milking machine-related damage. Large litter size (Cooper et al, 2016), and poor nutrition are known to be risk factors for mastitis in suckling sheep (Onnasch et al, 2002; Arsenault et al, 2008; Huntley et al, 2012; Grant et al, 2016).
Hyperketonaemia in late pregnancy increases the risk of mastitis as a result of Mannheimia haemolytica (Barbagianni et al, 2015). Orf virus infection results in denudation of the local lymphoid follicles around the teat, which increases the risk of mastitis (Mavrogianni et al, 2006; Fragkou et al, 2007). Dystocia is also a risk factor for clinical mastitis (Waage and Vatn, 2008). In grazing dairy ewes there is a positive correlation between higher faecal worm egg count and prevalence of subclinical mastitis (Kordalis et al, 2019).
Trace element deficiencies may also affect udder immune function: blood levels of selenium and vitamin A are lower in flocks with higher prevalences of clinical mastitis. Levels of selenium, vitamin E and A are lower in animals affected by mastitis than in unaffected animals (Giadinis et al, 2011).
Incorrect milking machine settings and maintenance will damage the teat, and increase the risk of mastitis (see later section).
There are heritable components to somatic cell count (Barillet, 2007), udder conformation (Casu et al, 2006; Crump et al, 2019), and so selective breeding for reduced susceptibility to mastitis is possible.
Staphylococci survive well on hands, so poor hand hygiene, and lack of glove wearing is a well-recognised risk factor for mastitis in dairy cattle. This also applies to sheep and goats (Vasileiou et al, 2018), and clinicians need to be aware that in many small herds/flocks routine hand milking will still occur.
Weather and climate will impact the risk of mastitis. Humidity will affect levels of environmental pathogens, while thermal stress can affect the susceptibility of animals to disease (Vasileiou et al, 2019a). Recent work in Greece has been able to model for the effects of climate, weather and altitude on mastitis risk (Giannakopoulos et al, 2019).
Overstocking (Caroprese, 2008) and poor shed hygiene, alongside concrete or exposed soil lying conditions (Cooper et al, 2016), increase the risk of mastitis. Flies can act as vectors of bacteria from infected to uninfected udder halves.
Diagnosis
The gold standard test for identifying the causal pathogen is bacteriological culture. However it is first important to diagnose those sheep or goats that are infected. Table 1 describes different grades of mastitis: clinical cases are thought to represent just 5% of in-fections (Bergonier et al, 2003), with the vast majority of infections being subclinical. However, many dairies do not routinely foremilk prior to cluster attachment, and so it is likely that grade I clinical cases are being missed. When carrying out a mastitis investigation, foremilking of every animal is essential to identify any with clots in the milk. In the absence of foremilking, farmers should check milk filters in the parlour. Another option would be to use in-line electrical conductivity, although this is less commonly installed.
Table 1. Mastitis grades
| Subclinical | No changes in milk/udder/behaviour |
| Grade I | Milk changes — clots, blood, abnormal colour |
| Grade II | Udder changes — heat, swelling, pain |
| Grade III | Systemic changes — pyrexia, depression, inappetence, behavioural change, recumbency |
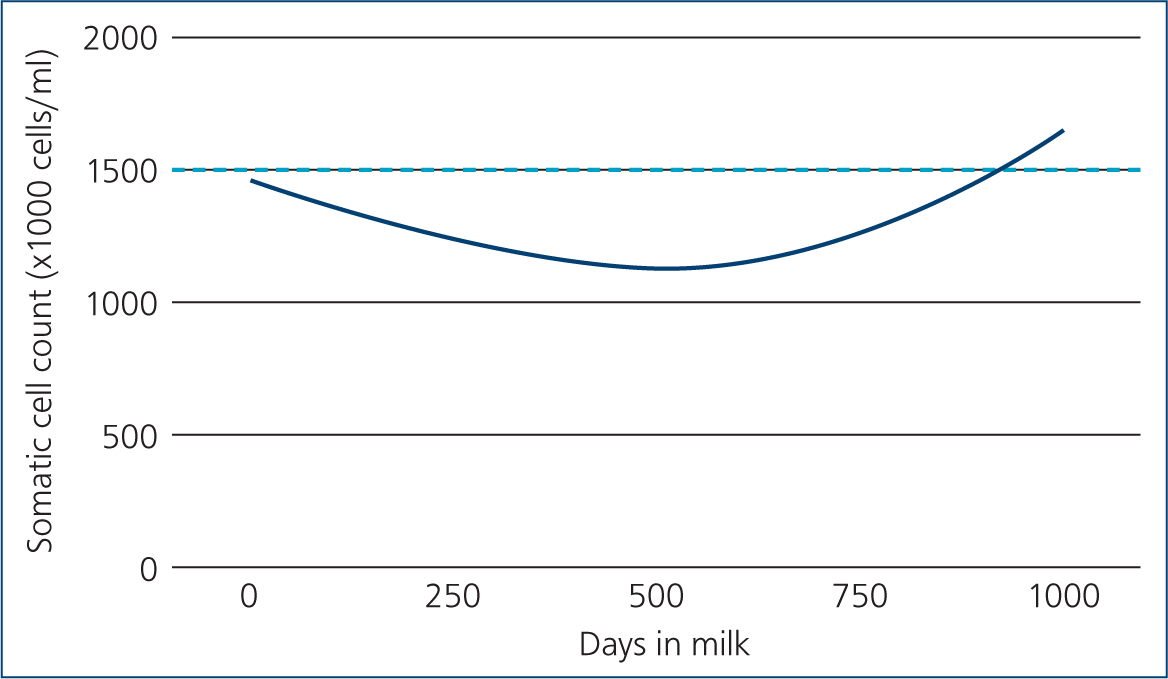
When investigating subclinical cases there is no visible change in milk quality. As a result of increased apocrine cell secretion, somatic cell counts tend to be higher in small ruminants than they are in cattle. Assessment of somatic cell counts can still be a useful way to identify subclinically infected animals, albeit with a higher threshold for classifying infection. One study showed that a threshold of 1 500 000 cells/ml had the greatest sensitivity and specificity for detecting goats infected with S. aureus (Koop et al, 2011). Sheep have slightly lower somatic cell counts than goats, and it has been suggested that a threshold of >1 000 000 cells/ml is more appropriate for sheep (Albenzio et al, 2012). These thresholds roughly translate to a score 2 or greater (distinct gel formation) in the California Mastitis Test (Contreras et al, 1996). The timing of somatic cell count testing is also important, as cell count rises during lactation (Wilson et al, 1995). Previous work has shown that the optimum time for sampling goats is between 40–150 days in milk (Moroni et al, 2005; Koop et al, 2012). Figure 2 shows how the average somatic cell count changes by days in milk in one goat herd milk recording with QMMS, relative to a threshold of 1.5 million cells/ml. Given the rise in somatic cell count in late lactation, goat herds that routinely milk for extended lactations (>2 years) are likely to have a higher prevalence of infection. In sheep the effect of days in milk is thought to be less important (Paape et al, 2007), although limited studies exist, particularly on UK farms.
It is rare for a mastitis problem to present as an outbreak of clinical cases. The more common scenario is for farmers to report a drop in milk quality or rise in bulk milk bacterial count/bactoscan. Generally the bacterial count of goat and sheep milk is higher than cow's milk, but bacterial counts of <20 000 colony-forming units (cfu)/ml are achievable (Table 2). Mastitis is a common reason for increased bulk milk bacterial counts; where the total viable count exceeds 20 000 cfu/ml, further investigation is warranted.
Table 2. British Sheep Dairy Association (BSDA) recommended raw milk standards
| ASPECT | BSDA TARGET | EU legislation | SCA regulations |
|---|---|---|---|
| Plate count at 30°C. Milk being pasteurised | <10 000 | ≤1 500 00 | <10 000 |
| Plate count at 30°C. Milk not undergoing heat treatment | <10 000 | ≤500 000 | <10 000 |
| or Bactoscan x1000 impulses/ml | <50 | Not specified | Not specified |
| Enterobacteriaceae/coliforms; cfu/ml | <100 | Not specified | <100 |
| Coagulase-positive staphylococci cfu/ml | <100 | Not specified | <100 |
| Somatic cell count/ml | <500 000 | Not specified | Not specified |
| Extraneous water | >540 and <570 | Not specified | Absent |
| Titratable acidity % lactic acid | <0.22–0.32 | Not specified | Not specified |
| pH value | 6.3–6.7 | Not specified | Not specified |
| Butter fat % | >5.0 | Not specified | Not specified |
| Protein % | >4.5 | Not specified | Not specified |
| Antibiotics | Pass antibiotic test | Must not exceed maximum residue levels | Pass antibiotic test |
| Temperature | <2.5°C holding temperature. On arrival at the dairy milk must not be above 10 | Milk holding temperature must not be above 8°C | On arrival at the dairy milk must not be above 10°C |
| EU regulations available at http://europa.euEU Regulation states that a rolling geometric average over a 2 month period, two samples a monthAttention should be paid to Specialist Cheese Association (SCA) standards as most milk is being processed into cheeseAs sheep milk is often bulked up for up to 4 days before collection, it should be stored below 2.5°C and samples taken for analysis at the time of collectionUnsatisfactory results; the milk buyer/processor should be informed of any results that are not in the target column, so that a sensible decision can be made regarding the usage of the milk | |||
| Unlike other countries there are no minimum standards for sheep or goat milk quality in the UK. Each processor sets their own standards, which vary considerably. These are the standards of the BSDA | |||
Control
As in cattle, infections can be acquired from other animals, i.e. ‘contagious’, or from the environment, i.e. ‘environmental’. Contagious spread occurs from animal to animal, most commonly in the milking parlour, or via suckling lambs/kids. Symptoms of a ‘contagious’ epidemiology include a high prevalence of infected or chronically infected animals, but a low rate of clinical mastitis. Environmental infections can be acquired at any time; symptoms of an ‘environmental’ pattern include a high rate of clinical cases, but good cure rates and fewer persistent infections.
Because of a lack of milk recording and clinical mastitis data, no large scale epidemiological studies have been carried out in the UK. However anecdotally, contagious spread is thought to be more common in small ruminants than it is in cattle (Bergonier et al, 2003). For this reason, control of contagious spread is often the first recommendation in the face of an outbreak. Attention to detail during the milking process is crucial in controlling new cases (Box 1 and Figure 3).
Box 1.Control of contagious mastitis
- Clinical cases should be identified as soon as possible and treated
- Milk infected goats/sheep last and disinfect clusters afterwards
- Use effective post-milking teat disinfection
- Minimise risk of fomite transmission, e.g. udder cloths, gloves, milking unit disinfection
- Avoid suckling kids/lambs
- Culling of persistently infected animals
- Regular milking machine maintenance including dynamic machine testing

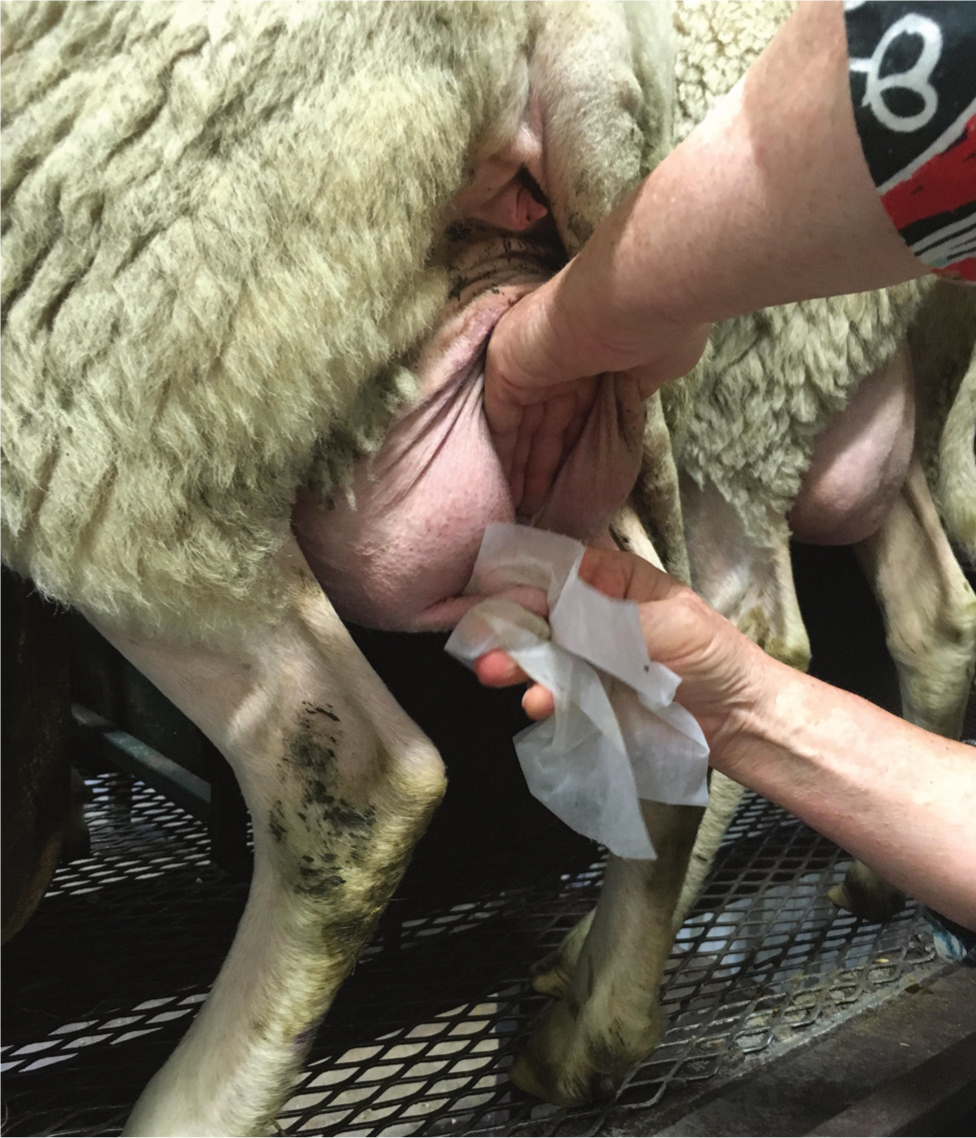
Management of environmental infections should focus on improving teat and udder cleanliness. Animals should be inspected for cleanliness away from milking (in housing or at pasture) and in the milking parlour prior to cluster attachment (Figure 4). Interventions around bedding management, improving ventilation and pre-milking routine are all likely to be important in controlling an outbreak of environmental mastitis (Box 2). Good access to loafing area, feed and water are also important to motivate animals to stay standing for 30 minutes after milking to allow teat sphincter closure.
Box 2.Control of environmental mastitis
- Increase bedding volume/frequency
- Where possible use inorganic bedding
- Reduce stocking density
- Adequate loafing area
- Good access to feed and water
- Improved management of water/wet areas
- Where grazing, ensure regular pasture rotation, e.g. 2 weeks on, 4 weeks off
- Improve ventilation
- Effective pre-milking teat and udder disinfection
Milking machine
Compared with cattle, goats and sheep carry a greater proportion of milk in the milk cistern rather than alveoli (Costa and Reinemann, 2004). This means that pre-milking teat preparation is more important for teat cleanliness than milk let down. Gross contamination should be removed as it has the potential to cause environmental mastitis or elevated bulk milk bacterial counts. Clusters are usually applied quickly, and without foremilking to save time. It is important that milking machines are correctly set up for different species. Compared with cattle, a lower system vacuum (37–38 Pa) is recommended, and a higher pulsation rate (90–120 pulses per minute) (Ohnstad; personal communication, 2021). Farms using an automatic cluster removal system should calibrate to a lower milk flow rate than in dairy cattle. Settings should be reviewed on a farm-by-farm basis, but a starting rate of 200 g/min + 10 second delay for goats, and 150 g/min + 10 second delay for sheep is recommended (Dzidic et al, 2019). In addition, post-milking teat disinfection is recommended in herds, particularly with a contagious aetiology. It is rarely used in small ruminant dairies, although it has been shown to reduce rates of new infection (Paape et al, 2001).
Lactating animal therapy
There are no licensed therapies for mastitis in small ruminants producing milk for human consumption. Prescribing veterinary surgeons should apply the cascade when making treatment decisions, by selecting therapies licensed in small ruminants for meat production or dairy cattle. Withdrawal periods are likely to be shorter or no different to those used in cattle (Karzis et al, 2007a, 2007b), although in the UK all off-license medicine use is subject to statutory milk and meat withholds (at least 7 days milk and 28 days meat). Where the infection is known to be subclinical, i.e. high cell count without visible milk changes, systemic treatment is not commonly recommended.
There are many licensed therapies for treating lactating dairy cattle, with both systemic and intramammary options available. In dairy cattle, there is limited evidence to support the use of systemic antibiotics in mild or moderate cases of mastitis. Furthermore, intramammary therapy constitutes a lower mass of antibiotic and should therefore be preferred. Given that most cases of mastitis are caused by Gram-positive bacteria, particularly Staphylococcus spp., treatments should be narrow spectrum where available. In the UK, narrow spectrum intramammary products are available containing penicillin and cloxacillin.
The use of intramammary preparations designed for cattle does pose some problems in small ruminants. The smaller size of the teats and streak canal relative to cattle is one: in order to avoid potential iatrogenic contamination of the mammary glands, it has been proposed to use partial insertion of the tip of the tube (Bergonier and Berthelot, 2003; Bergonier et al, 2003). One intramammary tube should be used per udder half.
Where Gram-negative species are suspected or have been identified on culture, broader spectrum intramammary tubes should be used. Non-steroidal anti-inflammatory drugs (NSAIDs) should be considered in all cases, especially where there is sign of grade 2 mastitis, i.e. inflammation, heat or swelling. When treating mastitis in dairy cattle, the addition of NSAIDs has been shown to have a net economic benefit, in terms of culling and reproductive performance (van Soest et al, 2018).
Where there is evidence of systemic infection, e.g. pyrexia or behavioural changes, broad-spectrum systemic therapy should be considered, as should NSAID administration, supportive care and oral or intravenous fluid therapy if signs of dehydration or shock are present. For more detailed guidance on the treatment of grade 3 mastitis cases, readers are referred to the accompanying article by Page et al (in preparation).
Dry period therapy
Given the increased prevalence of infection in late lactation, antibiotic therapy at drying off is recommended in known infected animals. Blanket antimicrobial therapy has been shown to reduce somatic cell count in the following lactation (Paape et al, 2001), although routine use of prophylaxis is discouraged by the British Veterinary Association, Responsible Use of Medicines in Agriculture Alliance (RUMA) and farm assurance schemes. Instead, intramammary antibiotics are recommended in known infected animals, this should include those that have recently had clinical mastitis or a high somatic cell count (diagnosed by milk recording or California Mastitis Test). It is also recommended that any animals with a diagnosis of S. aureus mastitis in that lactation should be given antibiotics at dry off.
Subcutaneous injection of tilmicosin has been reported to lead to a 43% decrease in mammary abnormalities during the subsequent lactating period (Croft et al, 2000).
Culling
Several criteria influence culling of ewes and does for mastitis control. Animals that have evidence of permanent damage to the udder, e.g. palpable lesions, should be culled, both because of the risk they pose to other animals as reservoirs of bacteria, and because of their reduced productive capacity. Grade 2 and 3 mastitis cases should provisionally be placed on the cull list, because of the likelihood of this damage occurring. Chronically infected and repeatedly relapsing animals should also be culled (Mavrogianni et al, 2011).
S. aureus in particular is capable of persisting in the udder for prolonged periods, both during and between lactations. Treatment during the lactation has a limited likelihood of clearing infection. Treatment in the dry period has higher success rates (Petridis and Fthenakis, 2014). Where animals have persistent high somatic cell count and infection from one lactation to the next, e.g. culture positive at the end of one lactation and early in the next, then consideration should be given for culling the animal. This is logistically easier to arrange and quantify for sheep than for goats, where extended lactations may occur. Penicillin-sensitive strains of S. aureus have a better prognosis for cure in cattle (Barkema et al, 2006), and penicillin sensitivity may also be a useful indicator in small ruminants.
Vaccine
There is a mastitis vaccine licensed for use in sheep and goats (VIMCO emulsion for injection for ewes and female goats, Hipra Uk & Ireland Ltd), which contains inactivated S. aureus. The full vaccination schedule should be completed before the expected parturition in order to offer protection from the beginning of the lactation period (i.e. when the mammary gland starts being at risk for developing mastitis). Milder clinical signs and reduction of the incidence risk of the infection has been reported in various studies. Vasileiou et al (2019a, b) described the efficacy of the vaccine in reducing the incidence risk of staphylococcal mastitis in ewes in an experimental and an extensive field study, indicating it offered protection against S. aureus and coagulase-negative staphylococci. A reduction in somatic cell count and infection rate, and an in-crease in spontaneous cure rate, is reported in vaccinated does compared with unvaccinated controls (Kautz et al, 2014).
In any case, vaccination should not be considered as the only means for controlling mastitis. Other udder health management measures should be implemented to improve control of the infection (Vasileiou et al, 2019a).
Conclusion
In order to advise clients well on the control of mastitis, both clinical and subclinical, in small ruminants, it is important that the differences between them and dairy cattle is recognised and understood. There is currently limited information about control of mastitis in dairy sheep and goats in the UK context, but there is an increasingly large body of evidence developing worldwide.
KEY POINTS
- Clinical mastitis is less common in small ruminants than cattle, although symptoms are often more severe.
- Subclinical mastitis is more common, and >90% of infections are caused by Gram-positive organisms.
- Mastitis outbreaks often present as elevated bulk milk bacterial counts.
- Somatic cell counts are often higher than in cattle, but can still be a useful tool for herd-level diagnostics.
- Contagious spread is more common, as such attention to detail during the milking process is important in the control of new cases.
- Therapy is challenging because of a lack of licensed products, but narrow spectrum intramammary products are recommended
- Vaccination can have a role to play in controlling S. aureus


