Infectious bovine rhinotracheitis (IBR) is a highly infectious disease of domestic and wild ruminants caused by bovine alphaherpesvirus 1 (BoHV-1). IBR has worldwide distribution with the exception of a limited number of countries which have successfully eradicated it (Ackermann and Engels, 2006).
In the UK, IBR is thought to be endemic, with studies published in 1998 and 2017 finding the proportion of herds with positive bulk tank milk to be 69% and 62%, respectively (Paton et al, 1998; Velasova et al, 2017). Another survey of suckler herds found 83% of 107 unvaccinated herds with one or more seropositive animals (Woodbine et al, 2009).
There are multiple drivers for the control and eradication of IBR, highlighting its contribution to economic, environmental and social sustainability, as well as trade implications. IBR is known to be harmful to bovine health and productivity and this is reflected in a negative impact on the farm-level profitability of dairy and beef production. This effect has been better studied in dairy farms. In Ireland, the IBR status of a dairy herd was found to have a significant effect on the performance and profitability characteristics of dairy farms (Sayers, 2017). The study showed that multiparous cows in bulk tank IBR-positive herds produced an average of 250 kg/year less milk than cows from negative herds. Other studies have linked prolonged reduction in milk production with sub-clinical IBR (Statham et al, 2015) as well as increased youngstock and cow culling rates and longer calving intervals (Raaperi et al, 2015).
IBR is part of the bovine respiratory complex and as such contributes to the usage of antimicrobials on cattle farms. Addressing IBR is therefore expected to contribute to reduced levels of antimicrobial usage and consequently counter the emergence of antimicrobial resistance. In addition, addressing IBR will lead to reductions in associated cases of illness and death, with consequent improvements in animal welfare.
In Europe, a growing number of countries and regions have approved IBR programmes or have been recognised as IBR-Free under the Animal Health Law (AHL; Regulation (EU) 2016/429 on transmissible animal diseases; Commission Designated Regulation (EU) 2020/689) by the European Commission. In both cases, the AHL lays down measures for the introduction of cattle into these countries and regions which have the potential to negatively impact live trade of cattle from other countries.
This article describes the disease caused by BoHV-1, its diagnosis and control and eradication at herd and European level.
The disease
Infected animals excrete high quantities of virus during primary infection. BoHV-1 is mainly spread directly by close contact between animals (Figure 1). It can also be shed from the reproductive tract, including semen, resulting in venereal transmission. Aerosol transmission typically occurs over short distances, but it may also occur over distances of up to 4.4 m (Mars et al, 2000). The virus is moderately resistant to environmental factors, so indirect transmission within or between herds can also occur through movement or sharing of contaminated facilities, equipment or personnel (Benavides et al, 2021).
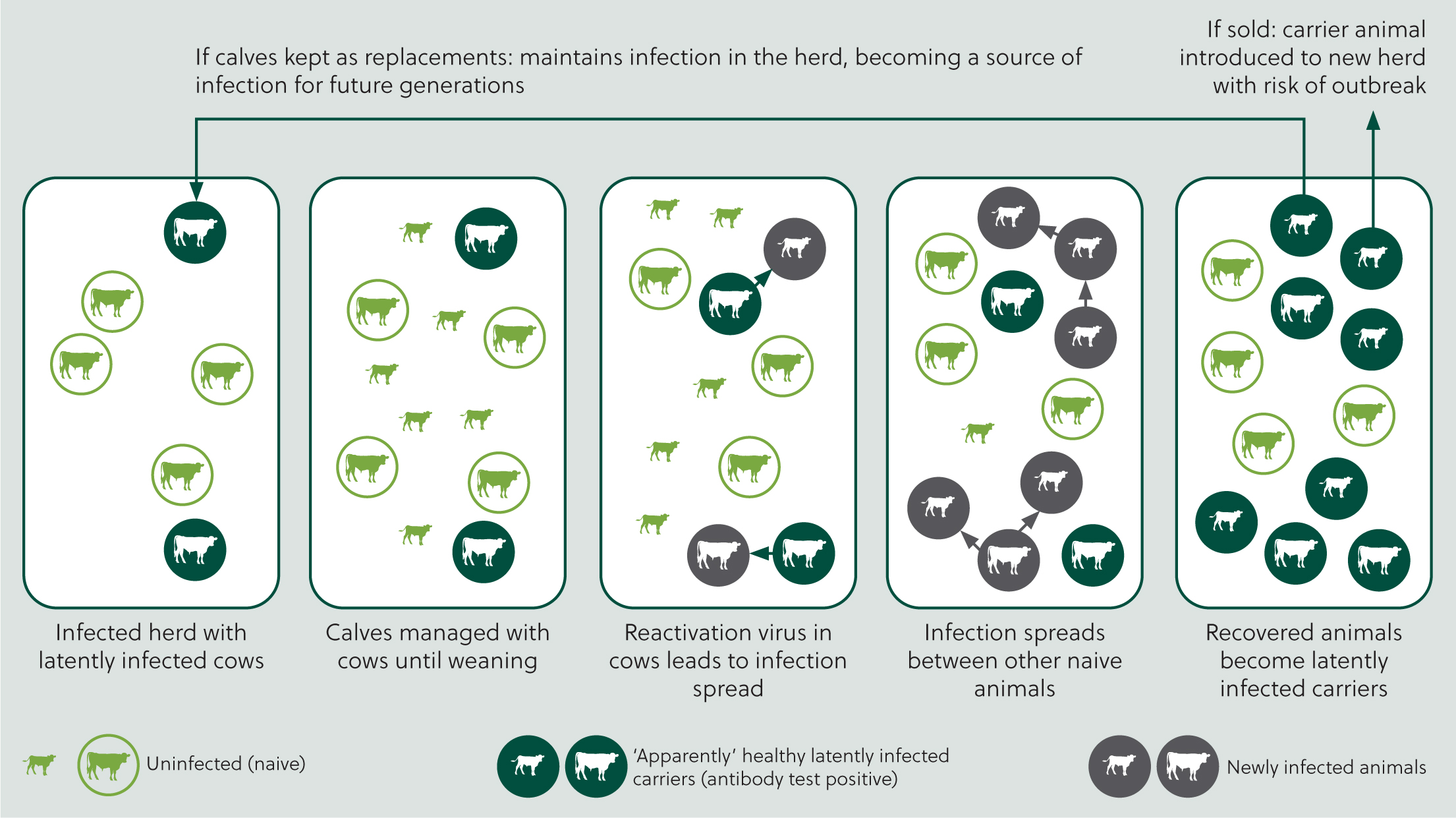
Clinical manifestations are variable, from inapparent to very severe (Wiseman et al, 1979; Pritchard et al, 2003), and dependent on viral and animal factors such as viral strain, viral dose, immune status and concurrent infections.
Clinical signs of BoHV-1 infection typically involve the upper respiratory tract and include nasal discharge, hyperaemia of the muzzle (red nose), conjunctivitis, fever, inappetence and, on occasions, death. This may be accompanied by decreased milk yields and a range of negative reproductive outcomes, depending on the stage of the reproductive cycle at which exposure occurs (failure to conceive, early embryonic death and abortion). Abortions associated with BoHV-1 are typically detected in the last trimester of pregnancy and the period of time between infection and expulsion of the fetus can be between 15 and 64 days (Crook et al, 2012). However, in some herds the course of infection can be sub-clinical but still associated with a reduction in milk yield and negative reproductive outcomes.
Recovery following initial infection is associated with the development of immunity, but this does not eliminate the virus. Instead, the virus establishes lifelong latent infection in the trigeminal ganglion or pharyngeal tonsils (Ackermann and Wyler, 1984). During this period the latent carrier is not shedding virus. However, at times of stress, such as transport, calving, mixing stock etc, the virus may be reactivated and can begin to multiply and be re-excreted, generally from the nose and eyes (Thiry et al, 1985). This leads to new infection in susceptible cattle, which in turn will also become latent carriers (Muylkens et al, 2007).
Diagnosis
Although infection with BoHV-1 can be suspected based on clinical and epidemiological findings, definitive diagnosis should be supported by laboratory examinations. For virus detection, nasal swabs from several affected animals in the early start of infection (ie serous discharge and pyrexia) should be collected and submitted for real time PCR testing (Nettleton and Russell, 2017).
The detection of antibodies to BoHV-1 is very important for the control and diagnosis of IBR. Since latently infected animals develop antibodies, the identification of serologically positive animals provides a useful and reliable indicator of infection status. Any animal with antibodies to the virus is considered to be a carrier and a potential intermittent excretor of the virus. However, non-infected cattle vaccinated with non-marker vaccines and calves with maternally derived antibodies will also be seropositive.
Two main types of enzyme-linked immunosorbent assay (ELISA) tests for the detection of BoHV-1 antibodies exist: conventional and marker tests. In the conventional kits, there are kits targeting the whole virus and kits detecting antibodies raised against the gB protein of the virus. Both will give positive results in animals that have been vaccinated with either conventional or marked vaccines, in addition to animals that have been infected (Table 1).
| Animal status | gE (marker) ELISA | Conventional ELISAs: whole virus and gB |
|---|---|---|
| Naïve | Negative | Negative |
| Infected | Positive | Positive |
| Vaccinated with conventional vaccine | Positive | Positive |
| Vaccinated with marker vaccine | Negative | Positive |
Marker tests detect antibodies to the gE protein (gE-antibody ELISAs). Animals vaccinated with gE-deleted marker vaccines can be discriminated from field-virus infected animals by a negative serological reaction for gE (Table 1). gE-ELISAs have been applied successfully in control and eradication programmes in several countries but have been shown to be less sensitive than the conventional ELISAs (Scientific Committee on Animal Health and Welfare, 2000).
Prevention and control of IBR
Plans for the control and eradication of IBR have been applied successfully (Iscaro et al, 2021). These are typically based on the identification and removal of lately infected animals, with or without marker vaccination. In infected herds, a well designed and implemented vaccination plan will reduce the likelihood of reactivation of latently infected animals, reducing within-herd transmission. It will also reduce the extent and duration of clinical signs in primary infections, reducing onward transmission from these animals. Over time, as latently infected cows are culled through routine management, and replaced by young, unexposed animals, the prevalence of infection in the herd will fall, potentially reaching zero depending on factors including the level of biosecurity and purchase policy.
Control at herd level
1. Plan
Herds will have different aims depending on the type of enterprise and their infectious status. Some herds will have bulls destined for EU-approved artificial insemination studs that should not be vaccinated, or cattle for export to BoHV-1-free countries that will not be allowed entry with any detectable antibodies to BoHV-1, including vaccinal.
2. Investigate the herd's IBR status
Herds with clinical IBR will have medium to high seroprevalence and are likely to already be implementing vaccination to control the clinical signs. For other herds, prior to the development of a control plan, is important to have an indication of the within-herd seroprevalence. Laboratory testing can be used to help decide what is the most appropriate control strategy.
Bulk tank milk testing
The use of bulk tank milk (BTM) as a herd level diagnostic tool has been applied extensively in IBR disease control programmes around Europe (Raaperi et al, 2014). When testing bulk tank milk samples, conventional whole virus ELISAs have shown the highest sensitivity and have been used extensively in non-vaccinating Nordic countries in their IBR eradication and surveillance programmes.
The gE (marker) ELISA in bulk milk gives a positive reaction only when more than 10–15% of the lactating animals contributing to the tank are infected, although the sensitivity can be increased by milk concentration protocols (Tignon et al, 2017). Herds with a positive gE BTM will be considered to have moderate to high prevalence and herds with a negative result, low to no prevalence. Therefore, this test is not typically used to declare a herd to be free from BoHV-1 infection. However, for general surveillance purposes, bulk milk tank tests can give an estimate of BoHV-1 prevalence in a herd, region or country.
Snapshot test
Beef herds can be initially screened by applying a herd ‘snapshot’, which requires the sampling of 30 randomly-selected animals over 9 months-old and testing them for gE antibodies (Graham et al, 2016). This sampling strategy has been applied before in other IBR programmes (eg, Belgium) as a cost-effective means to obtain an initial indication of the level of infection in a given herd.
If either no or only one animal is positive on the ‘snapshot’ test, the within-herd prevalence is estimated to be between 0–15%. If more than two seropositive animals are identified by the ‘snapshot’ test, the likely within-herd prevalence is greater than 15%.
3. Control
A control plan for a particular herd will have to be formulated according to the level of within-herd seroprevalence as indicated in Figure 2. There are three components in the plan: bioexclusion, to minimise the chance of the virus being introduced in the herd; isolation/culling of seropositive animals, which is only economically feasible in herds with low prevalence; and vaccination.
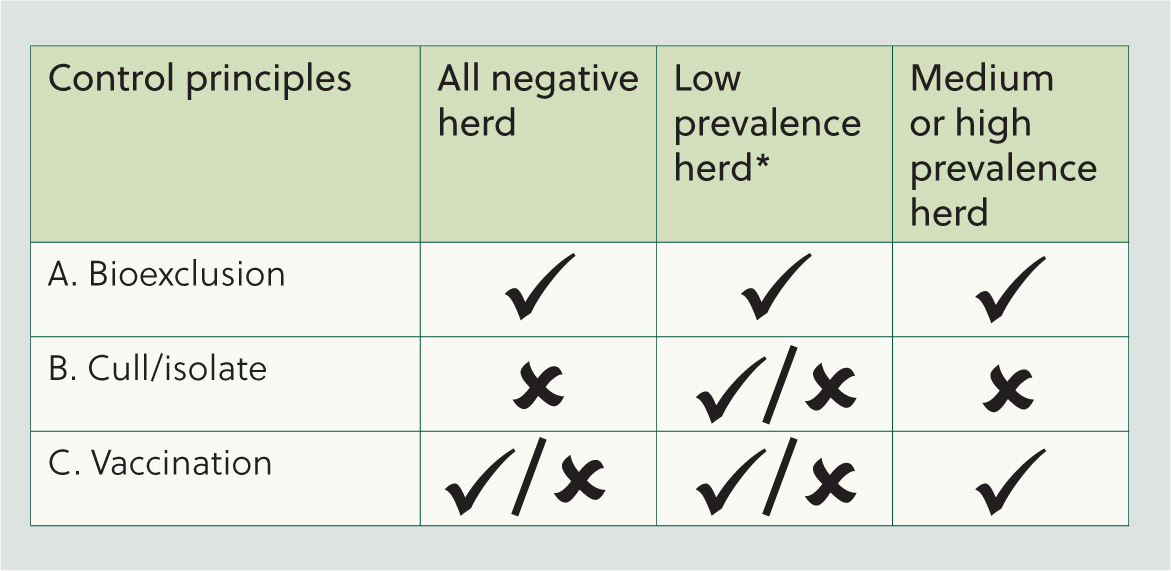
Biosecurity review
The biggest risk for IBR introduction comes from introduced animals (purchased, borrowed, animals returning from shows and sales etc). Therefore, closed herds have the lowest risk of introducing IBR, but if the farmer has to introduce animals, these should be isolated for 4 weeks and tested for IBR antibodies before they join the herd. Only negative animals should enter negative herds.
Avoiding mixing ‘home’ stock with cattle from other farms at pasture, housing or during contract-rearing will help prevent accidental introduction of infection. As the virus is also capable of being transmitted indirectly through equipment and people, it is important to maintain good hygiene of shared equipment and facilities, use separate clothing and boots or ensure appropriate cleaning and disinfection of boots and clothing.
Good building design, ventilation, stocking density, ensuring good nutrition and low stress environments are key to maintaining a healthy herd and minimising the risk of virus reactivation.
Herds with medium–high prevalence (>15%)
Non-vaccinating herds
Herds with a snapshot or BTM result indicating a within-herd prevalence of >15% that are not vaccinating for IBR should consider implementing a vaccination programme using a marked vaccine. A well-implemented vaccination plan that includes the whole herd will reduce within-herd transmission as mentioned above but it will also be important to review biosecurity to minimise the risk of virus reintroduction.
Vaccinating herds
Herds with a BTM result or a snapshot indicating a within-herd prevalence of >15% that are already vaccinating for IBR, should consider evaluating the efficacy of their overall control strategy (vaccination and biosecurity), taking into account the estimated prevalence and number of years vaccinating.
This should include quarantine and testing of added animals to ensure they are not carriers and strategic sampling of younger stock that should not have been exposed if vaccination and associated biosecurity is 100% effective. For example, in a herd that has been vaccinating for 5 years, all homebred animals less than 5 years of age should be testing negative.
Herds with low–no prevalence (0–15%)
Herds with a negative BTM or negative snapshot or with a single seropositive animal have a very low (or zero) true within-herd prevalence. Individual testing of all animals over 12 months or those not included in the snapshot would be required to confirm freedom (or to identify and remove any remaining carriers).
Again, these herds should review biosecurity, particularly in relation to added animals, to avoid accidental introduction. Marker vaccination should be considered but not necessarily introduced if the risk of introduction can be minimised with bioexclusion measures, noting that at this point the herd has avoided introduction of infection for many years.
4. Monitoring
All control programmes should be monitored regularly to review their effectiveness. Also, any suspicion of clinical signs of IBR should be investigated. Herds with low–no seroprevalence would need to confirm the herd status, which is best done by testing all animals or a statistically based sample from the herd, plus introduced animals.
In dairy herds, regular (3–4 times per year) BTM testing for BoHV-1 antibodies is a common monitoring method which may be done in addition to blood sampling of non-milking animals. In beef herds with medium to high prevalence, younger stock could be tested to check that those are uninfected and therefore the vaccination and biosecurity measures applied are being effective.
IBR eradication programmes in Europe
EU-approved programmes
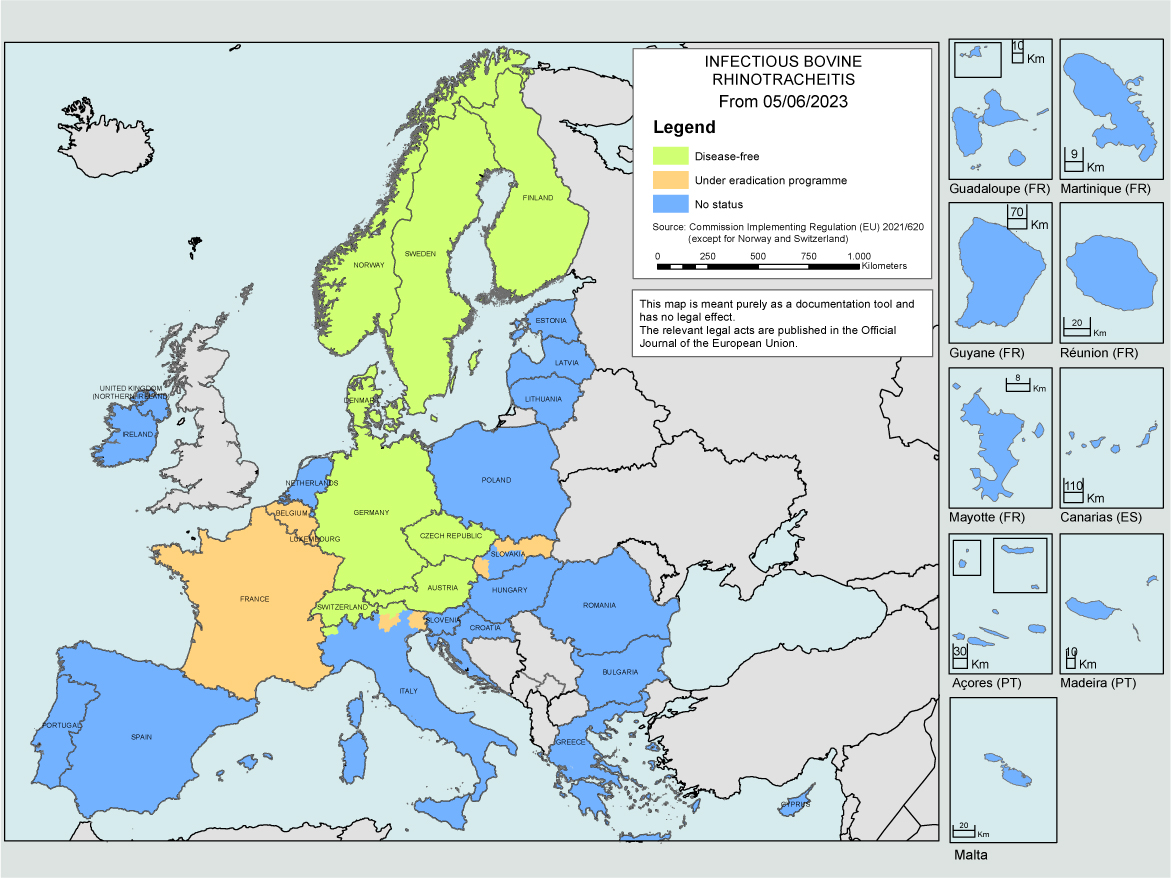
The EU has recognised IBR as a Category C+D+E disease (optional eradication in Member States) that needs regulating for intracommunity trade between member states. The first countries to obtain IBR-freedom were Denmark, Finland, Norway and Sweden. These are all now recognised as IBR-Free under the AHL. Austria, Germany, Switzerland and the Czech Republic, as well as regions of Italy are now also free of IBR (Figure 3; Table 2). In total, there are currently 14 countries or regions with additional EU guarantees for cattle trade, through having IBR-free status or an approved programme.
| Country | Beginning | End | IBR Free | Vaccination |
|---|---|---|---|---|
| Austria | 1987 Vol, 1990 Comp | 1999 | Yes – EU | No |
| Czech Rep | 2005 | 2020 | Yes – EU | No |
| Denmark | 1984 | 1992 | Yes – EU | No |
| Finland | 1978 | 1994 | Yes – EU | No |
| Germany | 1997 | 2017 | Yes – EU | Marker |
| Norway | NA | 1994 | Yes – EU | No |
| Sweden | 1994 | 1998 | Yes – EU | No |
| Switzerland | 1980s | 1988 | Yes – EU | No |
| Italy (regions) | 1990s | 2017 | Yes – EU | Bolzano - No |
| UK (Jersey) | NA | 2017 | Yes – EU | |
| Belgium | 1991 first Vol, 2012 Comp | Ongoing | No – Appr (2014) | Marker |
| France | 1996 Vol, 2006 Comp | Ongoing | No – Appr (2020) | Yes |
| Luxembourg | 2017 | Ongoing | No – Appr (2017) | |
| Slovakia | 1996 Vol, 2006 Comp | Ongoing | No – Appr (regions) (2023) | Marker |
| Hungary (region) | 2002 | Ongoing | No | Marker |
| The Netherlands | 1998, 2018 Comp for dairy | Ongoing | No | Marker |
Although most of these countries/regions achieved freedom with programmes based on identification and removal of seropositive animals and no vaccination, this can only be considered when the prevalence of BoHV-1 is very low. These countries typically started with programmes early on (Table 2), have lower cattle density than other countries, and managed to gather the necessary supports for embarking in control programmes at the time. Marker vaccines may be used within EU-approved programmes but for a herd to be considered IBR-free, as well as testing negative, it must not have been vaccinating for at least 2 years.
EU legislation as well as World Organisation for Animal Health (WOAH) standards cover the collection and processing of semen and embryos for international trade. Bulls entering semen-collection centres approved for intracommunity trade in Member States must meet quarantine and subsequent monitoring requirements (negative for all antibodies, therefore not vaccinated), with semen and embryos imported from third countries subject to similar requirements as described in EU Regulation 2020/686. Article 20 of this Regulation requires that prior to admission to quarantine, bulls come from an establishment that was free from IBR and have never been kept previously in any establishment of a lower health status.
Non-EU approved programmes
Outside of the AHL framework, the control of IBR is very heterogeneous due to the wide variation in disease prevalence, industry structure and government and industry support for its control within each region/country.
A recent review of control programmes for infectious diseases of cattle in Europe, which included data from 33 countries, found that 24 had IBR control or eradication programmes (Hodnik et al, 2021). Fifteen control programmes were compulsory and most (19) were implemented at a national level, with Italy, France, Portugal, Spain and Ukraine reporting regional programmes. Funding for these came from a variety of sources (private (43%), government (35%) or co-funded (22%)) and most aimed to control the disease.
In the UK, there are several cattle herd health schemes certificated by Cattle Health Certification Standards (CHeCS). These offer IBR control programmes for achieving and certifying freedom from IBR and provide a structured approach with or without the use of vaccines (CHeCS, 2023).
Discussion
Control and eradication of IBR both at herd and region/country level is possible. Even though evßidence-based best practices for the control and eradication of diseases are well understood, there is still a gap between those and what is implemented on farm. The need to understand both farmers' and vets' behaviours and their effect on disease control by using social science approaches is now increasingly recognised in veterinary epidemiology (Biesheuvel et al, 2021).
Over the last decades, there has been a growing realisation of the role of biosecurity as a key strategy for the prevention and control of diseases. However, several studies have reported a low level of implementation of biosecurity measures in cattle farming (Renault et al, 2021) and have explored farmers' motivations and barriers to behaviour change. Some reported the importance of farmers' perceptions of the efficacy or effectiveness of a biosecurity measure and the need for communication strategies focusing on the effectiveness to promote behaviour change (Renault et al, 2021). It is here where cattle veterinarians have the challenge to encourage their clients to engage with behaviour change to improve herd health. Evidence-based communication strategies which draw on psychological theory and practice have been shown to improve shared decision-making and collaboration between veterinary practitioners and their clients (Bard Id et al, 2022). These strategies are becoming more frequent as part of veterinary practitioners' CPD (https://www.veterinaryirelandjournal.com/large-animal/345-motivational-interviewing-in-veterinary-practice).
Conclusions
There are many reasons to control IBR, including improved animal health and welfare and its economic impact. One advantage in the control and eradication of this disease is that there are tools available that have been applied successfully in different countries and regions, so there are opportunities to learn from those experiences. The use of marker vaccines and associated marker tests provide an instrument for control of disease in herds with medium to high within-herd prevalence, but this strategy must be accompanied by biosecurity measures to minimise the risk of the virus being introduced in the herd. Control and eradication of IBR at herd level is achievable, but a systematic approach is essential to address disease control at a regional or national level.


