Nematodirus battus is a gastrointestinal parasite, which has a huge impact in UK sheep flocks as a result of lamb mortality and reduction in growth. Farmers and veterinary surgeons conventionally focus their efforts on preventing outbreaks of nematodirosis during the spring, but the incidence of autumn infections is increasing and becoming a growing concern. The aim of this article is to describe changes in the behaviour of N. battus and highlight important knowledge gaps with reference to informing effective and sustainable management.
N. battus is a common cause of enteritis, contributing to gastroenteritis in spring and early summer, typically seen in young lambs between 6 and 12 weeks old. The main clinical signs are profuse watery diarrhoea, accompanied by lethargy, loss of condition, dehydration and death. The acute onset of the disease is typically brought about by the ingestion of large numbers of the infective third-stage larvae (L3) having hatched en masse from overwintered eggs in response to rising temperatures (Figure 1). The presence of massive numbers of juvenile and adult worms in the small intestine causes physical damage to the villi and induces a catarrhal inflammatory process responsible for the clinical presentation (Figure 2). Summer-autumn infections characterised by diarrhoea and dags (dried faeces adherent to the wool of the tail and surrounding the perineal region) are also becoming commonplace (Figure 3); albeit their epidemiology is not completely understood.



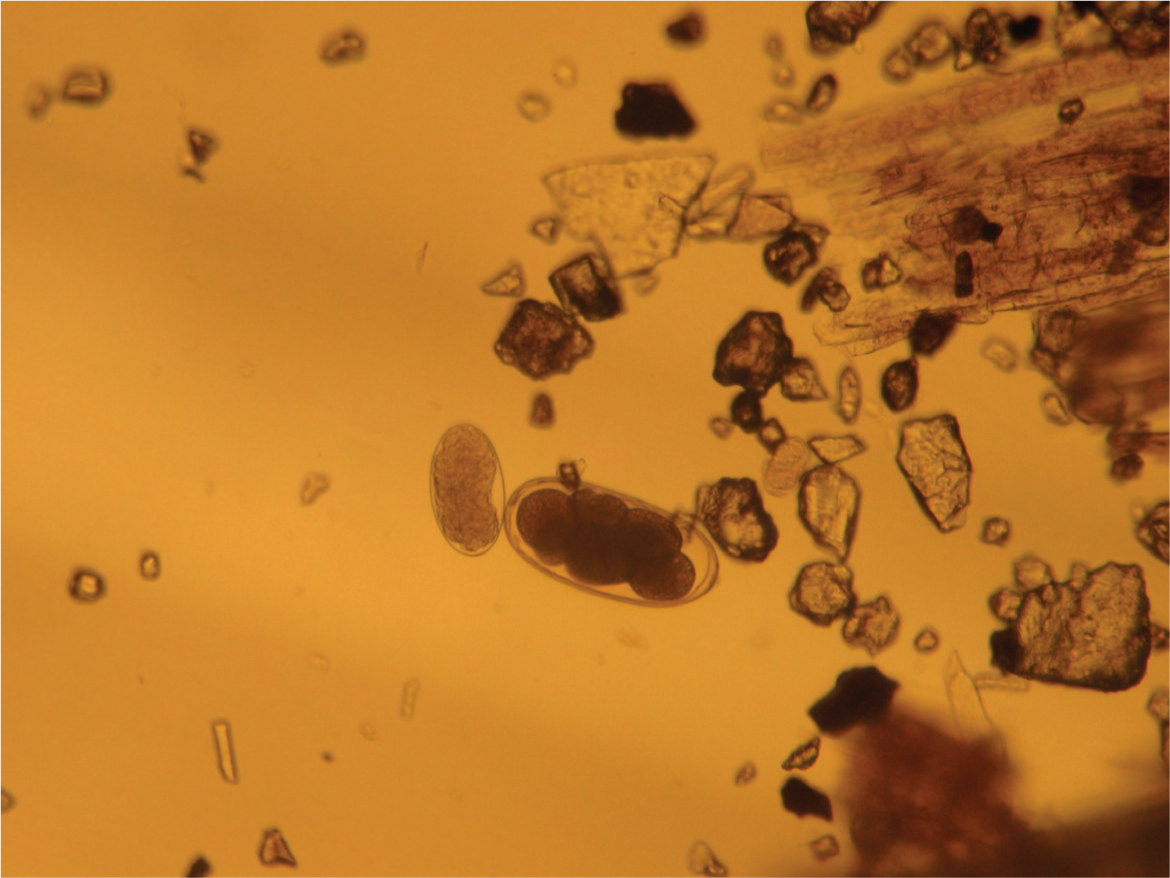
The diagnosis of nematodirosis is based first on the clinical, or post-mortem signs. Faecal egg counts (FEC) can be unreliable as a risk assessment and early diagnostic tool, because severe disease or deaths can arise in pre-patent infections. FECs are nevertheless helpful in less acute-onset cases and to monitor the impact of control strategies, or anthelmintic drug treatment efficacy. The eggs’ characteristic parallel-sided shape and large size relative to other trichostrongyle eggs, with blastomeres comprised of large dark, round cells (Figure 4) makes their identification straightforward.
Risk assessment programmes based on temperature data from weather stations around the UK (for example, https://www.scops.org.uk/forecasts/nematodirus-forecast/) have been developed to estimate the likelihood of nematodirosis occurrence in the spring at regional levels. The first models developed in the 1970s used mean soil temperatures, but later work has shown models that use air temperature to be more accurate in predicting the peak hatch of N. battus L3 (Hopkinson et al, 2021). These data, along with a history of grazing of the fields by young lambs during the previous year, are used to determine the need for and timing of anthelmintic treatments, but can lack sensitivity at individual farm level. In the face of parasite adaptation, highlighted by cases of autumn nematodirosis, preventive measures based on the traditional life history of N. battus may become ineffective. Detailed understanding of parasite epidemiology is, therefore, crucial to prevent disease outbreaks.
Historically, hatching of N. battus eggs was considered only to occur after a period of chilling followed by temperatures above 10°C (Smith and Thomas, 1972). Hatching still mostly occurs between 11°C and 17°C, and is markedly delayed at temperatures above or below these thresholds (Van Dijk and Morgan, 2010). Two types of eggs have been described, namely those requiring the classic chilling stimulus to hatch (chilling eggs) and those that do not (non-chilling eggs). The presence of the non-chilling eggs changes the hatching dynamics and creates a new transmission window. On most UK farms in a recent study, the numbers of hatched eggs have been shown to be higher with a chilling stimulus, but on some farms, independent of geographic location, non-chilling hatching is the predominant mechanism (Melville et al, 2020). Changes in the hatching patterns of N. battus are probably because of genetic adaptation to factors such as climate change, grazing management and farming practices.
Autumn nematodirosis may be caused by various interacting scenarios. A percentage of eggs deposited during the spring, or of eggs shed during the previous autumn, may hatch in the summer or autumn when the temperature falls back into the hatching range. These scenarios are dependent on specific climatic conditions, and if these are not achieved, larval emergence could be delayed by at least a year. The hatching of non-chilling eggs will be a source of continuous infection where the single synchronised spring hatch does not occur. This adaptation will reduce the risk of N. battus extinction in the face of unexpected environmental changes (Van Dijk and Morgan, 2008). Long survival of L3 in biomes where they are protected from ultraviolet light, heavy rainfall, or desiccation, such as rough grazing or environmental management zones (vegetation/habitats/field margins managed for environmental purposes), could also account for some cases of autumn nematodirosis (Sargison et al, 2012).
Each of the broad spectrum anthelmintic drug groups is licensed for the treatment of nematodirosis. The benzimidazole drugs have become the mainstay for N. battus treatments of lambs during the spring when anthelmintic resistant infections with other trichostrongyle species are considered to be unlikely. However, reports of benzimidazole resistance in N. battus (Mitchell et al, 2011) are becoming commonplace.
Genetic adaptations in N. battus, such altered hatching requirements and the emergence of anthelmintic resistance, represent a serious threat for sheep farmers and create a necessity to develop new strategies to reduce infective challenge and avoid further selection for resistance.
Case report
During the summer and autumn 2021, the FECs of lambs in a hill flock of about 500 ewes in the south-east of Scotland were monitored using a saturated saline and cuvette method with a detection threshold of 1 egg per gram (epg). N. battus and other trichostrongyle eggs were counted separately. The mean (±SEM) trichostrongyle and N. battus FECs on 5th August of 20 lambs from across the hill were 1187 (±121) and 140 (±20) epg. At this time, most of the lambs were showing signs of ill thrift, and all were weaned and treated with an anthelmintic drug at the time of weaning on 9th August. Cheviot, Scottish Blackface and smaller Mule ewe lambs were dosed orally with 7.5 mg/kg levamisole and moved to a silage aftermath field (G). Larger Mule ewe lambs were dosed orally at a rate of 7.5 mg/kg levamisole and moved to an in-bye field (C). Male Cheviot, Scottish Blackface and Mule lambs were treated with 2.5 mg/kg monepantel and moved to a silage aftermath field (B). In recent years, the silage aftermath fields had only been grazed during August and September by weaned lambs. The lambs that had been moved onto the silage aftermath fields grew well, despite many developing signs of diarrhoea and dags (Figure 5). The in-bye field had been grazed by ewes and lambs throughout the previous spring and summer. The lambs that had been weaned on to the in-bye field remained ill thrifty and developed signs of diarrhoea and dags. On 20th September, these Mule ewe lambs were dosed orally with 7.5 mg/kg levamisole and moved to another field that had not been grazed by sheep during the previous year. All of the lambs were sold off the farm during the first half of October.

The FECs of the three groups of lambs are shown in Figure 6. The efficacies of levamisole against trichostrongyles and N. battus were 98 and 97%, respectively in the G group and 100% in the C group. A single lamb in the G group had a positive post treatment FEC; and the shepherd reported that a few animals had sneaked past in the race and avoided treatment. The efficacy of monepantel against trichostrongyles and N. battus in the B group was 100%.
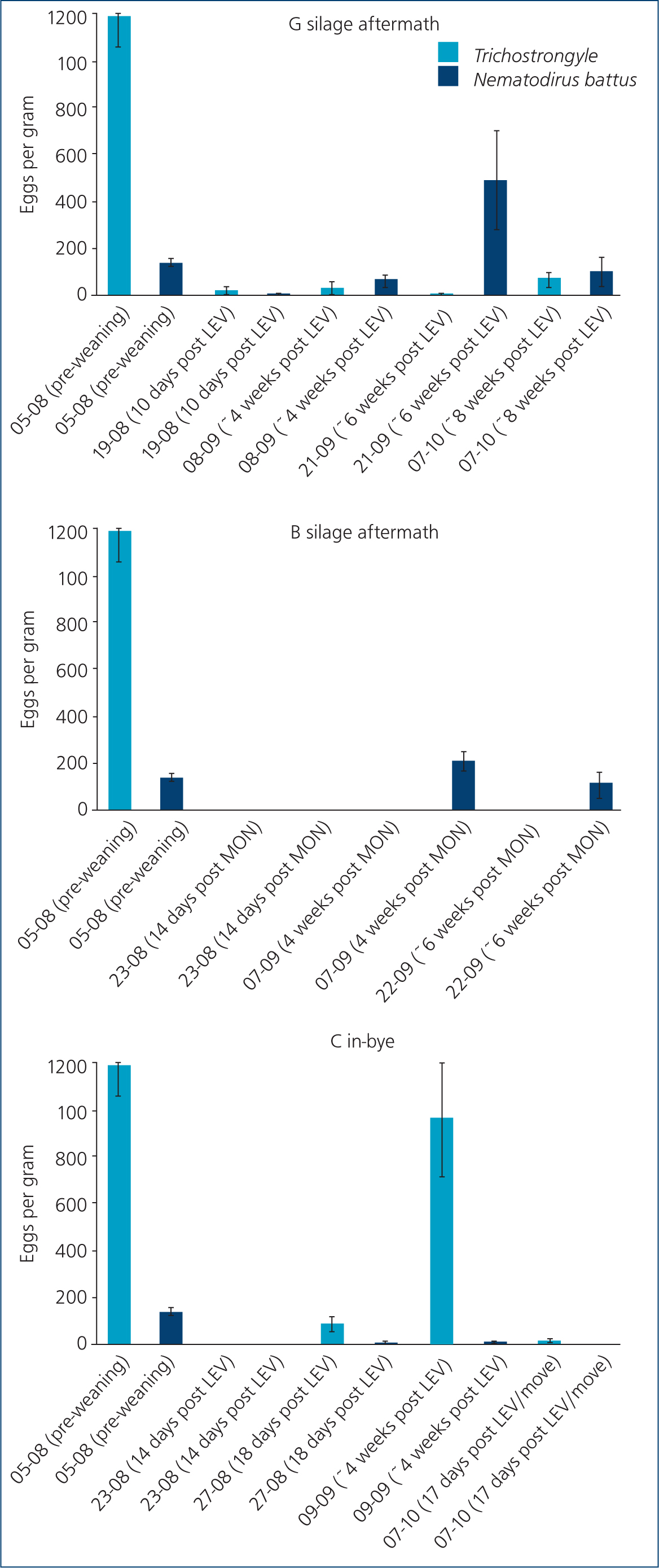
The trichostrongyle FECs of the lambs that had been dosed and moved onto the silage aftermaths remained very low. Positive trichostrongyle FECs in the G group of lambs were in single animals, and attributed to their having avoided treatment. By comparison, the trichostrongyle FECs of the C group of lambs that had been dosed and moved onto the heavily contaminated in-bye field increased from 18 days post-treatment to a mean of 969 (range 138–2862) epg by 4 weeks post-treatment. The N. battus FECs of both groups of lambs that had been dosed and moved onto the silage aftermaths increased from about 4 weeks post-treatment to a mean of 491 (range 3–1953) epg in the G group by 6 weeks post-treatment and 219 (range 36–360) epg in the B group by 4 weeks post treatment.
Ill thrift in the group of Mule ewe lambs that had been dosed and moved onto the in-bye field was attributed to high trichostrongyle worm burdens, having been acquired from the heavily contaminated pasture. The onset of N. battus FECs in the groups of lambs that had been dosed and moved onto the silage aftermaths corresponded with the onset of signs of diarrhoea and dags. Whole group anthelmintic treatments before moving lambs onto putatively clean silage aftermaths was considered to be responsible, because the fields will not be grazed for 10 months and will have two crops of silage taken after removal of the lambs; hence any resistant survivors of drug treatment are unlikely to survive. In this case, the only credible source of N. battus infection for the lambs that were moved onto the silage aftermaths would have been eggs shed by the previous season's lamb crop, which survived over winter and hatched during the autumn after the second cuts of silage. It is intriguing that the N. battus FECs remained low in the lambs that were grazed on the heavily contaminated in-bye field, implying little or no autumn hatching. There have been numerous reports of similar cases occurring throughout the UK during 2021.
Conclusion
This study highlights the need for and value of detailed clinical investigations in addressing important knowledge gaps pertaining to the development of effective and sustainable nematodirosis control strategies.
KEY POINTS
- Nematodirus battus is a common cause of gastroenteritis in young lambs across UK flocks.
- It is crucial to understand the multiple risk factors to reduce the apearance of an outbreak.
- The changing in hatching patterns increases the risk of new infections and clinical cases after spring and early summer
- These adaptations are likely to be driven by factors such as climate change, anthelmintic drug usage practices and farming practices.
- Further research needs to be done to fully characterise and understand the parasite epidemiology during summer and autumn.


