Adenoviruses are recognised by the poultry industry as the aetiological agent for several widespread and economically significant conditions, such as egg drop syndrome (EDS) and turkey haemorrhagic enteritis (THE). More recently, fowl adenovirus A serotype 1 (FAdV-1) has been identified as the virus most commonly associated with gizzard erosion and ulceration syndrome (GEU) and adenoviral gizzard erosion (AGE). Other FAdV strains (FAdV-8a, FAdV-8B and FAdV-11) have also been detected, albeit less frequently (Steer-Cope et al, 2017; Mase and Nakamura, 2014; Mirzazadeh et al, 2019). This article will focus on FAdV-1 as the primary aetiological agent of AGE.
Classification
Adenoviruses are medium-sized (70–100 nm), double stranded DNA viruses that do not have a capsule (Hess, 2000; The International Committee on Taxonomy of Viruses (ICTV), 2012). They are classified into five genera, two of which affect mammals (mastadenovirus) or fish (ichtadenovirus). The three genera that affect avian species include atadenovirus (which contains duck adenovirus type-1, responsible for EDS), siadenovirus (which contains turkey adenovirus A, responsible for THE) and aviadenovirus. The aviadenovirus genus currently contains nine recognised species, five of which are fowl adenoviruses (categorised A–E) and includes the strains associated with AGE (ICVT, 2012).
Adenoviral associated gizzard erosions were first documented in a flock of broiler chickens in Japan (Tanimura et al, 1993) and a number of subsequent cases were reported worldwide (Lenz et al, 1998; Abe et al, 2001; Garmyn et al, 2018; Grafl et al, 2018; Lindgren et al, 2022). The virus appears genetically identical in both Japan and Europe suggesting a common viral ancestor (Mase and Nakamura, 2014).
Clinical signs
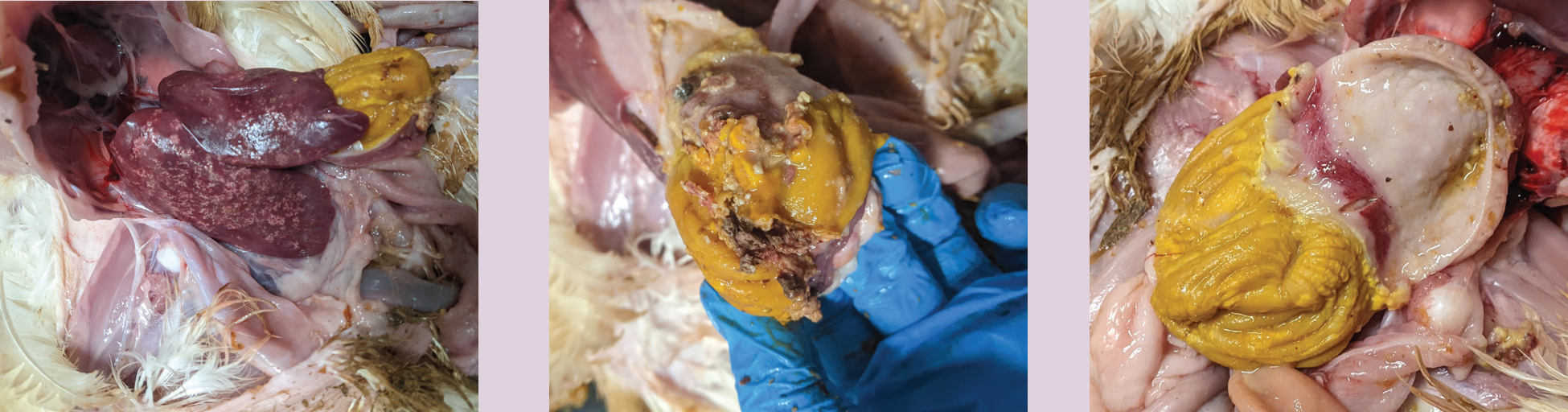
Gastrointestinal lesions predominate in cases of AGE, with the hallmark of the disease being severe erosions and ulcerations to the gizzard. As a result, clinical signs include poor weight gain, poor feed conversion, uneven growth and lethargy (Lenz et al, 1998; Okuda et al, 2001; Grafl et al, 2013, 2015; Lindgren et al, 2022).
Poor weight gain has been described in experimentally infected Ross 308 broilers and specific pathogen free (SPF) layers, with broiler weights 165.9 g less at slaughter compared with negative flocks (Grafl et al, 2012, 2022). Infected flocks in the field are also reported to have increased mortality, likely as a result of septicaemia as a sequelae to ulceration and bacterial translocation into the blood stream. There are cases described in which birds infected with pathogenic isolates of FAdVs have not shown clinical signs or variation in severity of gross lesions (Ono et al, 2001; Okuda et al, 2006). This highlights the potential for involvement of other factors in the clinical manifestation of AGE.
Fowl adenoviruses are well documented as having effects on both cellular and humoral immunity (Singh et al, 2006). The effect of immunosuppression on AGE has been explored in smaller studies, with evidence demonstrating FAdVs have greater effects in birds that are already immunosuppressed. However, further research would be required to understand how underlying conditions are linked to pathogenicity (Fadly et al, 1980; Muroga et al, 2006).
Transmission and disease process
FAdVs were first linked to vertical transmission back in the 1950s by Fry and Yates, horizontal transmission has since been experimentally confirmed (Yates and Fry, 1957; Ono et al, 2007). The role of vertical transmission is considered important in FAdVs and has been supported by several recent studies (Grgic et al, 2006; Grafl et al, 2012; Schachner et al, 2021). Since the initial case reports there has been evidence to support FAdVs being intermittently shed (Grgic et al, 2006; Mirzazadeh et al, 2021).
While infection may be vertical or horizontal, clinical signs generally do not begin until 10–14 post-infection, although signs occasionally occur as early as 5 days post-challenge (Ono et al, 2004; Okuda et al, 2006; Steer-Cope et al, 2017). There is minimal literature to fully ascertain how much protection maternally-derived antibodies (MDAs) provide to progeny, but some studies have shown high levels of MDAs did not protect progenies from development of gross lesions (Okuda et al, 2001; Ono et al, 2003). There may also be a link between viral load and the severity of detectable pathology (Ono et al, 2001, 2003).
Diagnosis
Histology is commonly used to diagnose AGE in field cases, as well as experimental studies. It benefits from being both practical and cost-effective. The gizzard lesions are characteristic, with degeneration of the koilin layer, basophilic intranuclear inclusion bodies, inflammatory cell infiltrate and degeneration of glandular epithelial cells (Ono et al, 2004; Grafl et al, 2012; Animal and Plant Health Agency (APHA), 2020). Histology along with concurrent clinical signs and post-mortem findings is currently used by the APHA as the standard diagnostic method for AGE (APHA, 2020). Due to the potential of immunosuppression leading to more severe clinical signs of AGE, bursal histology may also be evaluated at the same time.
Enzyme-linked immunosorbent assays (ELISAs) are available from several UK laboratories for group 1 adenoviruses, but are not specific for FAdV-1. For testing of FAdV-1 specifically polymerase chain reaction (PCR) can be utilised. Full genomic sequences for the reference FAdV-1 strain ‘CLEO Phelps’ and ‘OTE’ are used in most experimental studies to identify FAdV-1, although both are considered apathogenic in chickens (Okuda et al, 2006; Marek et al, 2010). Full genomic sequences of field strains causing AGE have also been completed, as well as creation of several phylogenetic trees (Mase and Nakamura, 2014; Matczuk et al, 2017; Lindgren et al, 2022). Cost, time and relatively low impact on clinical outcome are factors reducing the commercial use of PCR on a large scale.
The gross lesions of AGE appear to be limited to severe gizzard erosion, however viral detection via PCR shows evidence of viral colonisation of other tissues; namely the bursa and rectum. Viral isolation is also possible from pancreas, liver, thymus and kidneys (Okuda et al, 2006; Steer et al, 2015). At present it is unclear how viral colonisation of these organs may affect birds clinically.
The clinician should be aware that a visible gizzard erosion is not pathognomonic for AGE. Gizzard erosions are often found on post-mortem examination in birds with physical damage through litter-eating or inappropriate feed substrate. Some bacterial species have also been implicated in gizzard erosions and may have potential to exacerbate existing erosions through bacterial colonisation (Dinev, 2010). Gizzerosine is a toxin originating from fishmeal when it is cooked at high temperatures; it was first identified in the 1980s as a cause of gizzard erosions in broilers (Okazaki et al, 1983; Masumura et al, 1985). It has a biological action similar to histamine and causes a vast amount of hydrochloric acid secretion.
See Box 1 for a case diagnosis.
Box 1.Case diagnosisA flock of 42-day-old as-hatched broilers split over two houses showed poor weight gain for the previous 7–10 days. The weights were on target at thinning (partial depletion) (28 days: 1490 g). Litter quality declined post thinning with birds beginning to scour. Weights of the birds were markedly behind target at 38 days (2000 g). Mortality remained low despite the poor weight gain and at 42 days of age losses were 3.11%.On examination the intestinal faeces were pale and pasty and contained copious volumes of undigested feed. Close to 100% of the faeces were abnormal. Occasional black loose faeces was also noted. Environmental conditions and on-farm biosecurity were at a high standard. The birds appeared lethargic, some showing ruffled feathers. Feed and water consumption had been variable.A sample of birds were humanely culled via cervical dislocation for post-mortem examination. Birds showed severe gizzard erosions with necrotic tissue and proventriculitis. Some also showed hepatic necrosis.Due to the suspicion of adenoviral gizzard erosion (AGE), samples of the gizzards were sent for histological analysis at APHA Lasswade. These showed basophilic intranuclear bodies (Figure 1) and necrotising ventriculitis (Figure 2) which confirmed AGE in these broilers. A grossly affected liver was also sent for histology, which revealed hepatic necrosis.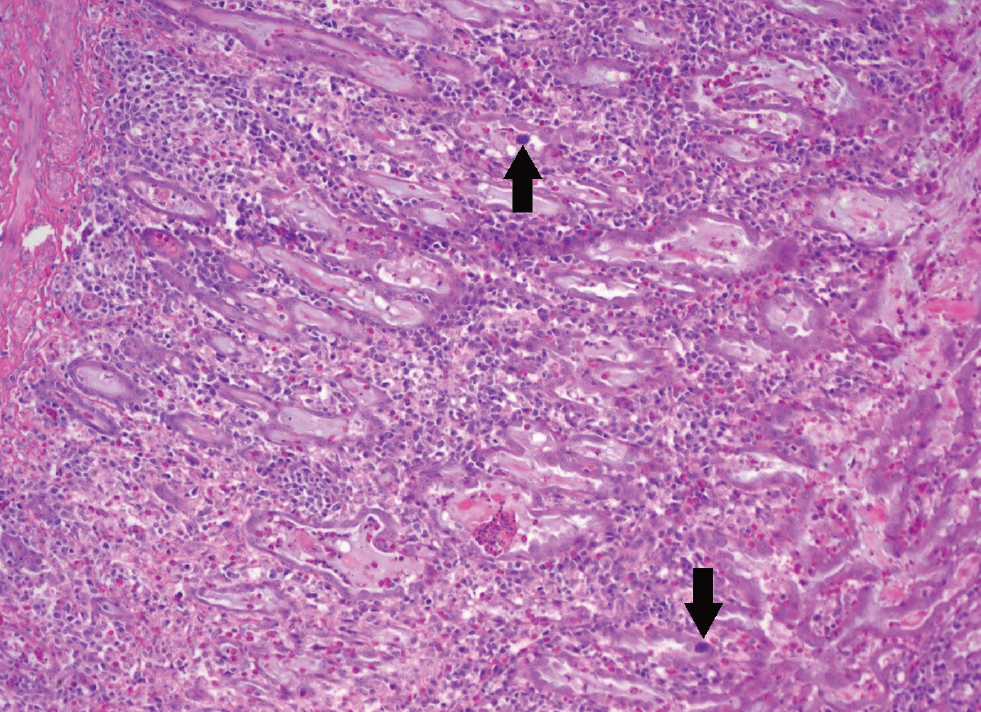
Figure 1. There are two basophilic intranuclear inclusion bodies in the glandular epithelial cells (arrows). There are mononuclear cells (round basophilic cells) infiltrating the mucosa and there are granulocytes (smaller eosinophilic cells). Photo courtesy of Christopher Poulos at APHA Lasswade.
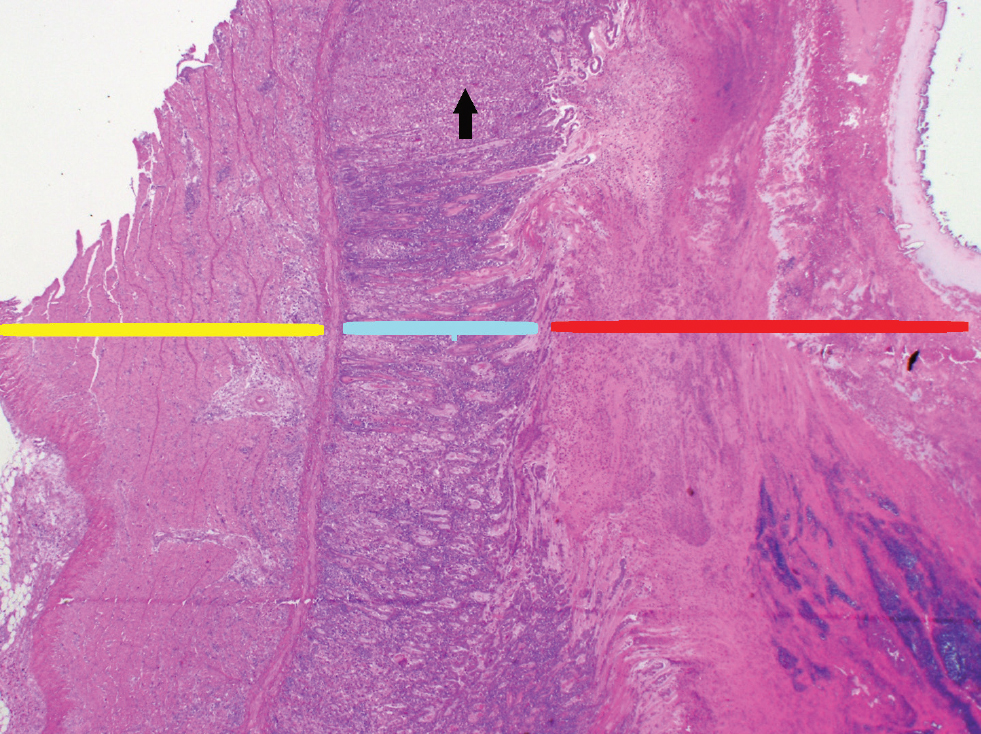
Figure 2. Necrotising ventriculitis. Tissue layers represented are the muscularis (yellow line), mucosa (blue line) and cuticle (red line). There are areas of glandular epithelium drop-out in the mucosa (black arrow highlights an example). The cuticle is expanded with cellular debris and haemorrhage. Photos courtesy of Christopher Poulos at APHA Lasswade.
KEY POINTS
- Adenoviruses are a large family of viruses that are responsible for a variety of diseases in poultry.
- Adenoviral gizzard erosion (AGE) should be a differential diagnosis in broilers experiencing poor weight gain; particularly when other conditions have been ruled out.
- Typical clinical signs include decreased weight gain, lethargy and maldigestion.
- On post-mortem examination, gizzard erosions are often seen grossly, histology should be utilised as a confirmatory diagnostic tool.
- Treatment is non-specific; including protecting birds from secondary infection and ensuring the immune system is not hampered by other stressors.
- Adenoviruses are robust; thorough cleaning and disinfection should be planned carefully to reduce environmental persistence.
Control
Control of AGE is complex, as there is still little information available on epidemiology of the disease within the UK. Varied presentation because of a range of co-morbities or absence of comorbidities, as well as variable clinical signs, may lead to underdiagnosis. It is unclear how much of a role FAdV-1 plays in the UK broiler industry, a recent report by the APHA found histological evidence of AGE in 8.9% of samples screened, however, these were screening samples and not suspect cases and therefore may not be a reliable indication of prevalence (APHA, 2020).
Experimentally, gizzard stimulating diets do not appear to impact the development of gizzard erosions (Grafl et al, 2015). Further studies would be needed to clarify if any dietary constituents are either beneficial or a risk factor in development of AGE.
There are no commercial vaccines for AGE currently available on the UK market. There has been some experimental evidence that is strongly suggestive of potential for birds to develop immunity to pathogenic isolates of FAdV-1 through the use of nonpathogenic strains, namely CLEO (Grafl et al, 2014). There is also evidence to show virulent strains of AGE inducing FAdV-1 become less pathogenic with consecutive passages through primary chicken embryo liver (Grafl et al, 2022). As with many endemic diseases the terminal goal is an effective universal vaccine.
Adenoviruses are robust and biosecurity is fundamental to reducing horizontal spread and transfer between units. Adenoviral gizzard erosions, like many other diseases, can be exaggerated by stressors including, concurrent diseases, diet changes and catching. Supporting birds through these challenges will help ensure the bird maintains a strong immune system, which may reduce the clinical effect of AGE in positive flocks.
Like other adenoviruses, FAdV-1 is resistant to many common disinfectants. However, glutaraldehyde with calcium hydroxide, formaldehyde, chlorine releasing agents, quaternary ammonium compounds, and sodium hypochlorite disinfectants are known to be effective against adenoviruses when used at the correct concentrations or an appropriate contact time (Furuta et al, 1976; Ishitaita, 2019; Inoue et al, 2020). Disinfectant use in the UK must comply with legislation, the Department for Environment, Food and Rural Affairs (Defra) produces and maintains a list of approved disinfectants and their dilution rates for use on poultry farms, this list should be referred to when choosing suitable disinfectants. The ability for FAdV-1 to survive many common disinfectants may lead to carry over from crop to crop and aid in horizontal transmission of disease.
Conclusion
Adenoviral induced gizzard erosions are seen in the UK. Diagnosis in the field is often successful with gizzard histology. Understanding the epidemiology can be challenging and treatment options are limited to the control of secondary infections and supportive care.


