The California mastitis test (CMT) is a cow-side milk test to detect the presence of subclinical mastitis. It is commonly used in the milking parlour to detect changes in the milk that are not visible to the naked eye. The test can be easily performed by a farmer or herdsman and gives a rapid result that allows a decision to be made about the milk hygiene before it enters the bulk tank. Standardisation of the test was published in the early 1960s to prevent confusion and misinterpretation of the test and this is still followed to the present day. At present the CMT is the only available cheap cow-side test for diagnosing the presence of infection in individual quarters. It is therefore widely used worldwide.
How to perform the California mastitis test
Milk is drawn from each quarter of the udder into a corresponding well in the paddle (Figure 1). Excess milk is drained away by tilting the paddle until approximately 3 ml of milk is left in each well, a graduated line on the rim of the wells denotes the level to drain to (Figure 2).
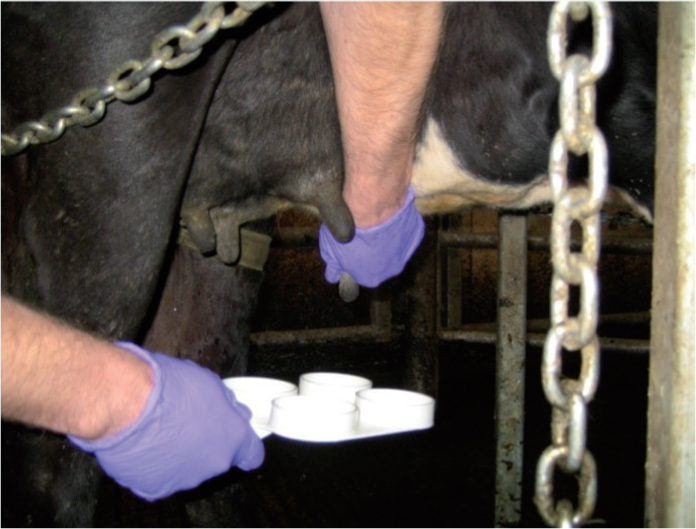

An equal volume of reagent is added to each well, using either a syringe or one pump of reagent from the supplied pump dispenser. The milk and reagent are mixed by allowing the fluid to swirl in the wells. The reaction score is recorded based on the observation of the fluid in each well compared with the standardised table aft er a mixing time of approximately 10 seconds (Figure 3; Table 1).
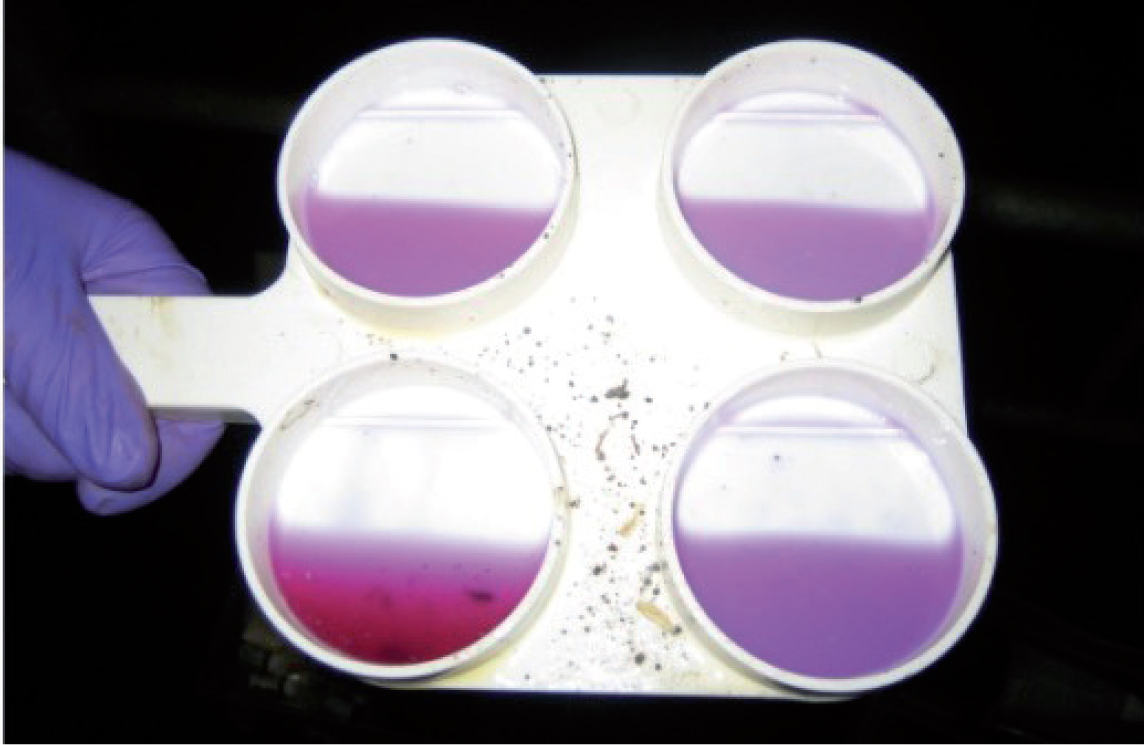
| Score | Reaction | Somatic cell count (cells/ml) |
|---|---|---|
| 0/-(negative) | Mixing remains liquid with no evidence of precipitate formation | 0–200 000 |
| T (trace) | Slight precipitate, which tends to disappear with continued movement of the paddle | 150 000–500 000 |
| 1 (weak positive) | Distinct precipitate but no tendency to gel formation | 400 000–1 500 000 |
| 2 (distinct positive) | Mixture thickens immediately with some suggestion of gel formation. As mixture is swirled, it tends to move towards the centre, leaving the bottom of the outer edge of the cup exposed. When the motion is stopped, the mixture levels out again, covering the bottom of the cup | 800 000–5 000 000 |
| 3 (strong positive) | Distinct gel formation that tends to adhere to the bottom of the paddle and, during swirling, a distinct central peak forms. | Over 5 000 000 |
| + (alkaline milk) | Plus sign is added to score when mixture is distinctly purple, as indicated by contrasting deep purple colour | |
| Y (acid milk) | letter ‘y’ is added to score when mixture appears yellow |
Based on the stated somatic cell count (National Mastitis Council, 2021)
History of the California mastitis test
The CMT was developed in 1957 by two American scientists in California (Schalm and Noorlander, 1957). These scientists began by working on the field Whiteside test, which involves adding 2 ml sodium hydroxide (NaOH) and 1 drop of 1–300 bromocresol purple to 10 ml milk. There was visible gelling of a healthy sample in the test, which reduced its specificity. By looking at several Whiteside reactions under a microscope, the scientists determined that it was the milk fat globules formed following the addition of NaOH that produced this result. The NaOH was therefore removed from the test procedure completely and a number of detergents trialled instead. The detergents that were useful for the reaction were the sodium and potassium salts of long-chain fatty acids, alkyl sulphates, alkyl sulfonates, alkyl arylsulfates or alkyl arylsulfonates. The reagents in a 3–5% concentration reacted with the milk of high cell count in a manner that could be graded. With the anionic detergents being of neutral pH, an indicator such as bromocresol purple could be added to increase the contrast of the colour when revealing abnormal alkalinity or acidity of the milk. This only needs to be at a ratio of 1:10 000 with the detergent to provide a contrasting colour against a white background. The diluted CMT reagent prior to use should be purple; if this is not the case then it should not be used. The reagent can be affected by the quality of water used to dilute the concentrated reagent, particularly if hard water is used. For this reason, most commercially-available CMT kits come with the reagent already diluted (1 pint concentrated reagent to 7 pints bottled water) and ready to use. The indicator is yellow at pH 5.2 and turns purple with neutral or alkaline milk. The optimum detergent for the test was the anionic surface-active agent, alkylary sulfonate. First identified in 1954, this is now a widely-used chemical used in detergents and emulsifiers. The detergent enhances the release of the abnormal materials in the mastitic milk and prevents the gelling of the milk that occurred through prolonged contact with the NaOH. The research pair, Schalm and Noorlander (1957), then developed a plastic paddle with four receptacles (cups) to hold the milk samples, the design of which is almost identical to that still being used today.
The test relies on three principles:
Organic debris, for example dirt or manure, that does not contain any DNA, will not interfere with the CMT reaction, thereby making the test easy to carry out even in a busy milking parlour. Once the first few squirts of foremilk have been discarded, the next 15–20 ml are representative of the total milk in the udder, meaning the CMT can be used to represent a measure of somatic cell count (Gray and Schalm, 1960). Again, this is a simple and easy procedure to carry out witin the milking routine without disturbing the milking process.
The CMT reagent contains water, sodium alkyl sulfonate (NaC12H25SO4, 3%), indicator colour (bromocresol purple, 1:10 000) and preservative (chloroacetamide). It was found that the use of domestic detergents instead of the commercially-available CMT reagent was investigated in 2008 (Leach et al, 2008). The cheaper detergents contain lower concentrations of anionic surfactants (5–15% compared to 15–30% in more expensive detergents). The cheaper detergents were less likely to produce a reaction with lower somatic cell count samples. A sensitive operator was able to use diluted Fairy Liquid to identify infected quarters with a sensitivity and specificity comparable to the CMT reagent (82 v 84% sensitivity and 93 v 91% specificity) for milk samples over 200 000 cells/ml.
The accuracy of the California milk test
The first work describing the accuracy of the CMT demonstrated that 95% of quarters reacting to the CMT reagent were shedding bacteria of a pathogenic nature (Marshall and Edmondson, 1962), with errors quoted to be 32% (Philpot, 1969). There was also a ‘broad’ correlation with the number of inflammatory cells found in the milk. Since this work, there have been numerous studies that have reported on the accuracy of the test, but these values vary depending on the gold standard used for comparison and, in some cases, the threshold used for interpretation. Table 2 summarises the accuracy of the CMT as described in the literature.
| Accuracy | Threshold used | Comparison | Reference |
|---|---|---|---|
| Sensitivity 97% | Trace | Somatic cell count >500 000 cells/ml or bacterial culture (major pathogens) | Barnum and Newbould, 1961 |
| Sensitivity 78% Specificity 76.1% | Grade 1 | SCC >100 000 cells/ml for positive, SCC <250 000 cells/ml for negative | Ewbank, 1962 |
| Correlation with SCC 52% | Grade 1 | SCC >400 000 cells/ml | Postle, 1964 |
| Sensitivity 84% | Grade 2 | SCC >500 000 cells/ml | Aynsley and Buol JM, 1965 |
| Efficiency 69.5 | Grade 1 | Bacterial culture (any organism) | Cole et al, 1965 |
| Correlation with SCC 85% | Trace | SCC >500 000 cells/ml | Smith and Schultze, 1965 |
| Sensitivity 89.9% | Grade 1 | SCC >500 000 cells/ml | Miller and Kearns, 1967 |
| Sensitivity 90% | Grade 1 | Bacterial culture (any organisms) | Brookbanks, 1996 |
| Specificity 70% | Trace | Bacterial culture (any organisms) | Philpot, 1969 |
| Sensitivity > 90% | Grade 1 | SCC >500 000 cells/ml or bacterial culture (major pathogens) | Green, 1984 |
| Sensitivity 66.7% Sensitivity 49.5% | Trace | Bacterial culture (major pathogen) Bacterial culture (minor pathogen) | Sargeant et al, 2001 |
| Sensitivity 82% Specificity 81% | Trace | Bacterial culture (major pathogens) | Dingwell et al, 2003 |
| Sensitivity 70% | Grade 1 | Bacterial culture (major pathogens) | Ruegg and Sekito, 2004 |
| Sensitivity 95.2% Specificity 68.5% | Grade 1 | SCC >100 000 cells/ml | Hogeveen, 2005 |
| Sensitivity 46.3% Specificity 81.9% | Grade 2 | Bacterial culture (major pathogens) | van Werven, 2005 |
| Sensitivity 87% Specificity 89% | Grade 2 | SCC >400 000 cells/ml | Leach et al, 2008 |
| Sensitivity 82% Specificity 98% | Grade 1 | Bacterial culture (major pathogens) | Safi et al, 2009 |
| Sensitivity 52.4% Specificity 83.7% | Grade 1 | Bacterial culture (all pathogens) | Godden et al, 2017 |
Operator variability
Operator variability is inevitable due to the subjective and qualitative nature of this test: however, this has not been investigated extensively. One paper demonstrated that the test sensitivity between operators varies more than the test specificity, resulting in under-detection of samples with a high somatic cell count (Leach et al, 2008). The operators, whether experienced or inexperienced, had 80% agreement when scoring samples as ‘high’ (score 2 or 3) or ‘low’ (score 0, T or 1). Overall, this means that decisions based on the CMT result will vary between operators on farm and between farms. This is a weakness of the test and its application on farm, but the effect can be minimised by following the testing procedure and grading criteria.
Quantification of the test
Quantification of the test is likely to be the main method by which to overcome the limitations of the test while still maintaining its practicality and convenience. The methods by which the test can be quantified rely on the characteristics of the fluid at low cell counts and the gel formed at higher cell counts, through knowledge of fluid dynamics and, in particular, the viscosity of fluids. The first attempt to quantify the viscosity of milk with the CMT reagent was in 1964, using a direct falling-ball viscometer to measure the fall time of a ball bearing in the milk and reagent mixture. The technique was modified 2 years later and described as a suitable method to determine the viscosity of the CMT gel by several research groups (Milne, 1977; Milne and De Langen, 1977; Duirs, 1980). The method correlated highly with the DNA content of the milk samples (r=0.94) and the New Zealand group described the rolling-ball viscometer technique as a practical method for measurement of the DNA or somatic cell count of tested milk samples (Milne and De Langen, 1977). However, its practicality was limited due to the complicated measurement technique and delicate nature of the equipment, making it inappropriate for use in the milking parlour environment.
The first use of an electroviscometer was the Fischer electroviscometer, with the maximum viscosity taken to be the viscosity measurement. This gave good reproducibility and was deemed a suitable instrument for subclinical mastitis detection (Nageswararao and Calbert, 1969). This same research group considered milk protein to play some role in the reaction and published work demonstrating that soluble casein increased the viscosity of the gel in the CMT reaction. However, the group studying the rolling-ball viscometer in 1977 experimented with adding sodium caseinate, bovine albumin and globulin to the milk and found the influence of added protein was not significant with no alteration in the developed viscosity of the reaction (Milne and De Langen, 1977). This demonstrated that milk quality did not affect the outcome of the test, again substantiating the usefulness of the test for subclinical mastitis detection.
An in-line somatic cell count sensor is available in New Zealand and the UK, based on the addition of CMT to milk before its gel reaction being assessed via the time taken to flow through an outflow orifice (Whyte et al, 2004). The company that produced this sensor has given an overall sensitivity of 96% for the device, although to the present date there are no formal data published in peer-reviewed literature to substantiate this fact.
The threshold of the test
There is little published work reporting the precise threshold at which the CMT reaction occurs. It appears that the generally-assumed threshold of 300 000 cells/ml originated from the work that described the optimum sensitivity and specificity of the test compared to somatic cell count measured by other methods. There are publications describing the CMT threshold at various levels (Table 3).
| Test | Threshold of somatic cell count | Reason | Reference |
|---|---|---|---|
| CMT | 200 000 cells/ml | Assumption | Schepers et al, 1997 |
| CMT | 200 000 cells/ml | Assumption | Leach et al, 2008 |
| CMT | 386 500 cells/ml | Compared with SCC and manual CMT | Amaral and Ruegg, 2004 |
| CMT | 500 000 cells/ml | Compared with microscopy and manual CMT | Barnum and Newbould, 1961 |
| CMT | 340 000 cells/ml | Grade 1 compared with microscopy | Luedecke et al, 1967 |
Rotational viscometry has been used to evaluate and quantify the test (Whyte et al, 2005); this has formed the basis for some of the automation of the test for inline use. The author has also done work using the same method and quantified the CMT using rotational viscometry and found a threshold of between 377 000 and 383 000 cells/ml (Roberts, 2023).
Advantages of the test
Limitations of the test
Practical applications and the future of the California mastitis test
The CMT continues to be used in many different situations, with key opinion leaders globally stating that it:
‘is the only reliable screening test for subclinical mastitis that can be easily used at the cow-side’
‘is arguably the only reliable screening test for subclinical mastitis that can be easily used cow-side’
‘CMT gel has the potential to be used as the basis of a reliable on-line sensor for estimating the SCC of milk’
There are tests available for use in conventional milking parlours or in an automated milking system that rely on the CMT. At present. There is limited validation of these devices and while the CMT reaction is quantifiable, it is not easy or straightforward and the threshold of detection is not known. Calibration of the devices is also challenging (Neitzel et al, 2014). A validation study for the in-line system used in an AMS system found that the in-line CMT and somatic cell count had 0.53 correlation, with a higher correlation at higher somatic cell count (particularly >500 000 cells/ml), (Deng et al, 2020). The main draw-back with this device was that the CMT reagent could run out, without notification to the farming staff. The use of automated CMT devices for mastitis detection should be done with caution, while it can be an additional tool for detection of clinical mastitis cases, as a standalone detection test, it has significant limitations on most farms, particularly when used on composite milk samples.
Practical uses of the California mastitis test: an evidence based approach
1. Identification of infected quarter following composite somatic cell count result
Appropriate use. The threshold at the individual quarter level for classifying quarters as infected is 400 000 cells/ml (Mollenhorst et al, 2010). The CMT, when performed correctly, is likely to have high sensitivity and specificity to identify the infected quarter.
2. Identification of high cell count cows and quarters
Appropriate use on individual quarter samples. The threshold at the individual quarter level for classifying quarters as infected is 400 000 cells/ml. Use of the CMT on composite samples is inappropriate as the threshold on a cow level is too low (usually 200 000 cells/ml or lower).
3. Confirming presence of clinical mastitis
Appropriate use. Most clinical cases of mastitis will have a cell count in excess of 400 000 cell/ml at both cow and quarter level. The sensitivity and specificity of the CMT at this threshold will still be adequate for this purpose.
4. Monitoring cell count post-treatment of mastitis
Appropriate use on individual quarter samples. Although a negative CMT result may demonstrate an improvement in a quarter case of clinical or subclinical case of mastitis, a full cure of an existing infection would require the cell count to be below 200 000 cells/ml at the cow level. An individual quarter CMT result can therefore be an aid to monitoring post-treatment, but a composite sample has less value.
5. Identification of infected quarters for bacteriological evaluation
Appropriate use. Bacteriology is optimally performed on infected quarters, ie those with a cell count in excess of 400 000.
6. Checking of individual cow's composite or quarter somatic cell count status prior to drying off
Not appropriate for use on composite samples. Cows should be treated at drying off according to their cell count, with high cell count cows receiving antibiotic dry cow therapy to optimise cure of existing infection for the dry period than those with low cell count. This is usually performed using a threshold of 200 000 cells/ml at the cow level.
Appropriate use on quarter samples. The addition of the CMT to drying off protocols can aid selective dry cow therapy decision making by allowing an additional check for infected quarters at the time of dry off. This is particularly useful where a complete set of milk records for a lactation is unavailable. It is also useful in herds that have had problems with performing selective dry cow therapy, such as a high dry period infection rate or post-infusion clinical mastitis cases, and want to increase confidence that they are minimising the risk of missing an infected cow so that they can maximise cure rates in the dry period. This may mean that more cows are dried off with ADCT due to the high false positives described earlier: however, as an additional tool at dry off to encourage selective dry cow therapy, it may be beneficial on some farms. Similarly, for farms that have had post-infusion infections following dry off, the addition of the CMT at the point of dry off alongside a review of drying off technique can increase confidence in continuing with selective therapy. In herds that do not milk record, the CMT can be an valuable, accessible tool in helping aid decision making at dry off. The CMT should be carried out in accordance with the standard procedure and interpreted using the recognised grading system – this can produce a sensitivity of 86% for major pathogens with a negative predictive value of >95% in low prevalence herds (Sanford et al, 2006).
7. Checking of new calved cows to establish somatic cell count status entering lactation
Appropriate use on individual quarter samples. The use of somatic cell count testing with a threshold of 400 000 cells/ml on individual quarters or 200 000 cells/ml on composite samples on day 4 or 5 post-calving has the optimum sensitivity and specificity for detecting intramammary infection (Sargeant et al, 2001; Dingwell et al, 2003).
8. Checking of purchased cows to monitor somatic cell count status
Appropriate use on individual quarter samples. The threshold at the individual quarter level for classifying quarters as infected is 400 000 cells/ml. Inappropiate use on composite samples as the threshold at the cow level for classifying a cow as infected is 200 000 cells/ml. This should be an aid to herd screening and should not replace other aspects of surveillance or biosecurity with incoming stock.
The distinction between correct use of the CMT at the individual cow level (composite samples) or individual quarter level is important to allow results to be interpreted correctly. In general, the CMT is performed manually at the cow-side using the paddle to select individual samples for evaluation. However, it is possible for farmers to use any milk sample for the CMT and the newer in-line devices that rely on the CMT reaction are placed within the milking line to analyse only composite milk. Composite samples can be used to aid with the detection of clinical mastitis cases, particularly when used alongside other detection methods (eg in automated milking systems) but the threshold of detection is too high for them to be a valuable aid for detection of subclinical mastitis. Quarter level testing can aid in decision making on farm and be a valuable and easy addition to aid mastitis diagnosis and support mastitis control programmes.
Conclusions
The California mastitis test can add value to many herds by aiding in the detection of subclinical mastitis in dairy cows. Its use should be considered for identifying individual quarters of high cell count cows, to identify high cell count quarters when milk recording information is limited or to aid in selective dry cow decision making in some herds. The limitation of the test is that the threshold is around 400 000 cells/ml and that repeatability between observers can be poor. This article presents the published work on the use of the test and discusses the benefits and limitations of using the test in various herd circumstances. As a affordable, readily available and simple to use cow side test, it can be complimentary to other tests to aid in mastitis control in many dairy herds.


