In recent years, genetic progress has accelerated, largely because of advances in the genomic testing of bulls (Guinan et al, 2023). This has shortened the generation interval and increased the rate of genetic improvement (García-Ruiz et al, 2016). Bulls are now marketed based on genomic breeding values before any phenotypic data are available from their progeny. Another significant development is the improvement in the quality and availability of dairy-sexed semen, allowing farmers to be more selective about which animal replacements are bred from. Conception rates to sexed semen have improved and are now between 83% and 100% as effective as conventional semen (Crites et al, 2018; Oikawa et al, 2019; Irish Cattle Breeding Federation, 2023).
Data quality is also improving. In the UK in 2023, 20% of heifer registrations were genomically tested, representing a significant increase in recent years (Agriculture and Horticulture Development Board (AHDB), 2024). This compares with 20–25% of heifers registered in the US(CoBANK, 2024).
These advancements create more opportunities for veterinarians and consultants to engage with clients regarding breeding decisions. Proactive herd health advice should include both genetic and management guidance (Egan, 2024).
How genetic information is used
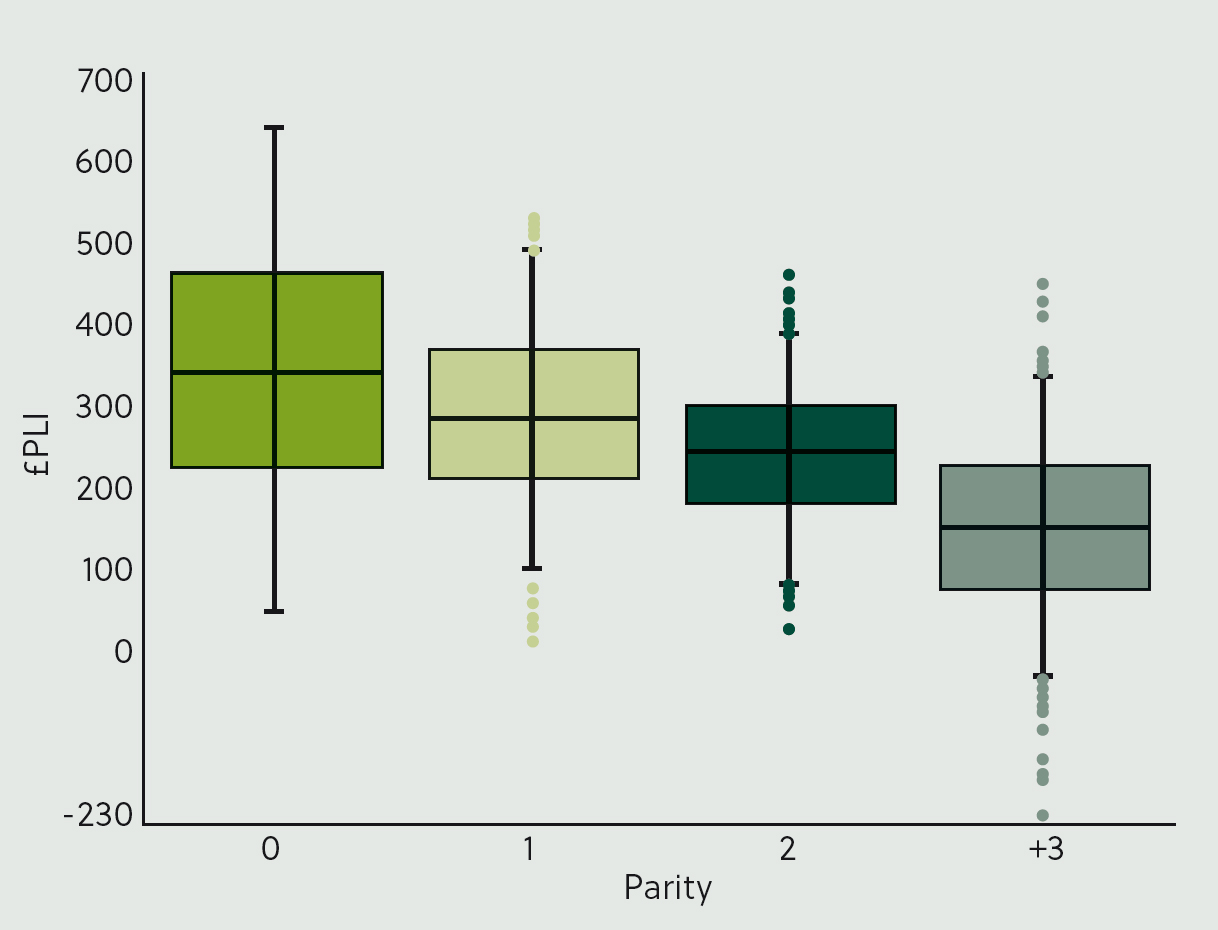
If breeding is progressing in the right direction, the youngest animals should have the highest genetic merit (García-Ruiz et al, 2016). However, there is likely to be variation across the population. Figure 1 shows the distribution of profitable lifetime index (£PLI) across different parities in an example herd. The median £PLI is highest in parity 0 animals; however, many older cows have a higher £PLI than the average heifer. In this example, the top 17% of cows have a £PLI equivalent to the top 50% of heifers, suggesting that the optimum breeding strategy should source replacements from a combination of heifers and cows.
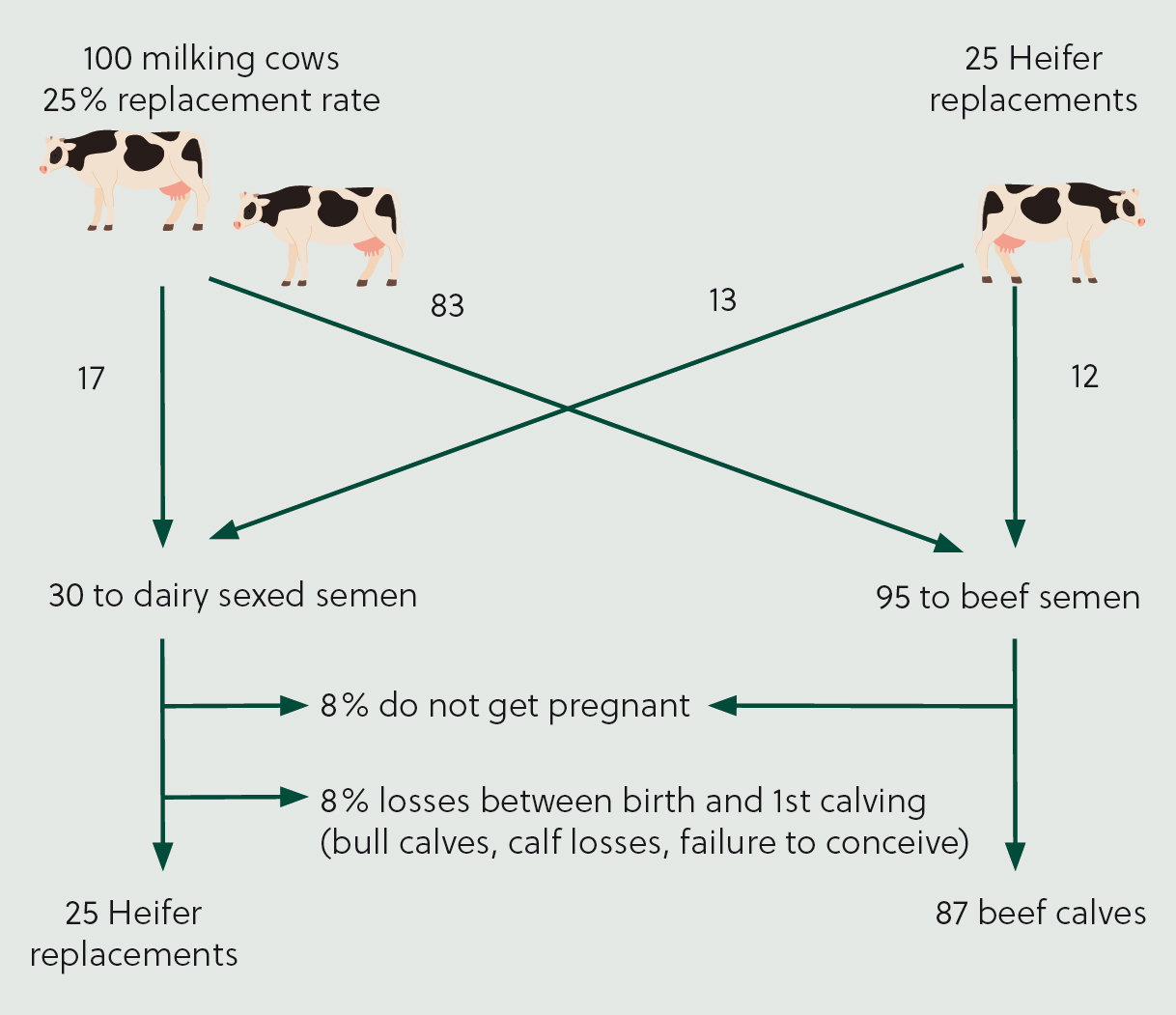
Once females with the highest genetic merit have been indentified, it is cost-effective to breed these animals to dairy-sexed semen, with the remainder bred to beef semen to produce higher-value calves. Figure 2 illustrates how the optimum genetic breeding strategy could be applied in a hypothetical 100-cow herd. This results in 25% heifer replacements, with approximately 50% of heifers and 83% of cows bred to beef.
On farms with very high genetic merit, the very best cows and heifers can be used as embryo donors, with embryos transferred to animals of lower genetic merit.
Parent average vs genomic breeding values
There are two types of breeding values available in the dairy industry: parent average (PA) and genomic. Parent average breeding values use pedigree information, including data from the sire, dam and their pedigrees. Genomic requires a tissue or hair sample, from which DNA is extracted, and sections of the genome are compared to a reference population.
Both PA and genomic values are improved over time as phenotypic data are fed back. Advances in record-keeping and more accurate dairy herd improvement testing have enhanced data quality in the UK. Accuracy is further improved by international data sharing (Winters et al, 2024), with the best results seen in large populations such as Holsteins (VanRaden and O'Connell, 2018). To maintain high accuracy, it is crucial that farmers continue to test and record on-farm performance (Gonzalez-Recio et al, 2014; VanRaden et al, 2020; Winters et al, 2024).
The key difference between PA and genomic results is reliability in early life. A newborn heifer can expect 20–25% reliability of breeding values based on PA data, compared with 65–70% for genomic testing. Throughout an animal's lifetime, PA estimations improve, provided production data are available. For herds that regularly conduct milk recording, PA values tend to achieve a similar level of reliability to genomic testing by the 4th or 5th parity. However, by this stage, farmers have already made up to five breeding decisions using less reliable data.
Another disadvantage of PA is the potential for missing or inaccurate pedigree information. Genomic testing can identify animals with incorrect parentage records and match them to the correct sire. In the UK in 2024, 17% of genomically tested Heifers had incorrect or missing sire information (AHDB, 2024). In such cases, PA calculations would have been incorrect or unavailable.
Understanding composite indices
Composite indices combine desirable traits into a single score, enabling farmers to rank cows and bulls on a consistent scale. These indices allow selection for multiple desirable traits simultaneously, while minimising undesirable ones. Although milk production constitutes a large component of these indices, fertility and health traits are also included.
In the UK, the most commonly used index is the £PLI. Bespoke indices have also been developed for block calvers, such as the autumn calving index (£ACI) and spring calving index (£SCI). Other countries use their own composite indices, for example, net merit (NM$) in the US.
Cost-benefit of genomic testing
The cost-benefit of genomic testing depends on individual farm factors, including the quality of pedigree information available for calculating PA (Weigel et al, 2012). The benefit is greatest in heifers, as the reliability improvement from PA to genomic testing is highest in this group (Weigel et al, 2012). Testing heifers also provides farmers with the maximum opportunity to make informed breeding decisions. The cost of a genomic test is relatively small over the lifetime of an animal, and in most scenarios, farmers are likely to see a net benefit over the life of a heifer (Weigel et al, 2012; Newton et al, 2018).
Many farmers may prefer to rely on PA results, but this approach may not result in the optimum breeding strategy. Figure 3 shows the correlation between parent average and genomically calculated £PLI for a group of heifers. The correlation is 28%, indicating weak agreement at the population level. When selecting the top 50% of animals to breed from, the average £PLI differs depending on whether PA or genomic testing is used:
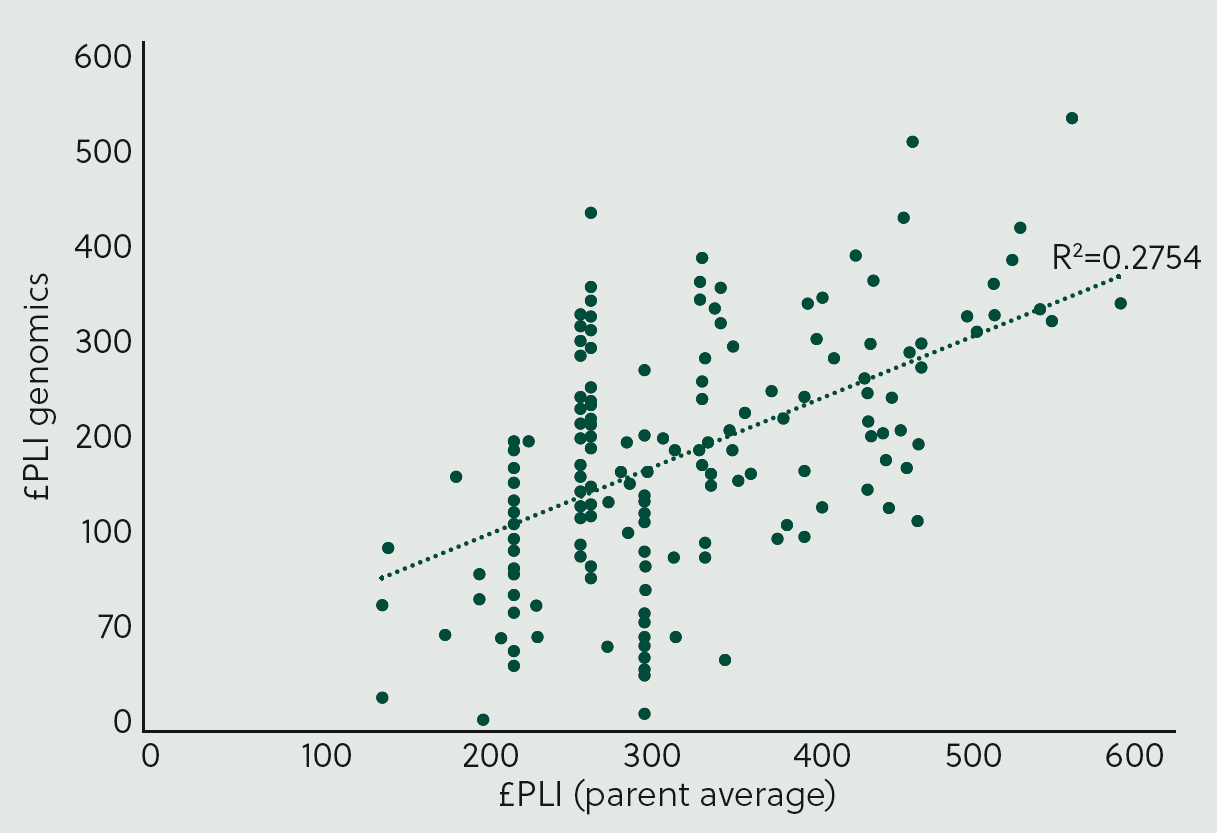
This translates to a 57-point advantage in £PLI for every heifer bred in that year. It is estimated that each point on the £PLI scale equates to a net margin of £1.58 over that animal's lifetime (AHDB, 2021). Since heifers inherit half their genetic potential from the dam and half from the sire, an additional 57 points of £PLI should translate to a £45.03 benefit per heifer replacement in the current breeding year. This profit is likely to compound annually, as future replacements will be bred from animals with progressively higher £PLI, resulting in an accelerating rate of genetic progress. This increased value aligns with findings from a UK case study (Lloyd et al, 2020), which reported a benefit of £49.89 per tested heifer. Given the average cost of genomic testing, typically £25–30, most farmers would see a net benefit from testing heifers.
Additional benefits of genomic testing, though harder to quantify, may include screening for genetic diseases or undesirable recessive traits. Some laboratories also offer BVD antigen testing, which can support disease surveillance.
Assessment of genetics and performance
The primary drivers of £PLI, £ACI and £SCI are milk production (yield or solids), fertility and health. Therefore, it is crucial to assess how effectively these genetics are being expressed within a herd before selecting bulls based on a composite index. Analysing performance data alongside genetic data can reveal whether genetics are being expressed and, more importantly, identify areas where they are not. If genetics are not being expressed, this indicates a bottleneck in farm management that requires attention.
Milk yield
Milk production is highly heritable (Schneider and Van Vleck, 1986), and selection for this trait has been a focus of breeding programmes for decades. However, management also plays a significant role in determining a cow's ability to produce milk.
Figure 4 presents data from an example herd, showing the correlation between genetic potential for milk yield and 305-day milk production in the most recent lactation. Heifer lactations are shown in red and cow lactations in blue. The line of best fit for heifers indicates a positive association, with better genetic potential linked to higher milk production. The gradient of the line provides insight into how effectively genetics are being expressed, while the R2 value reflects the strength of the relationship between genetics and performance, akin to a heritability estimate.
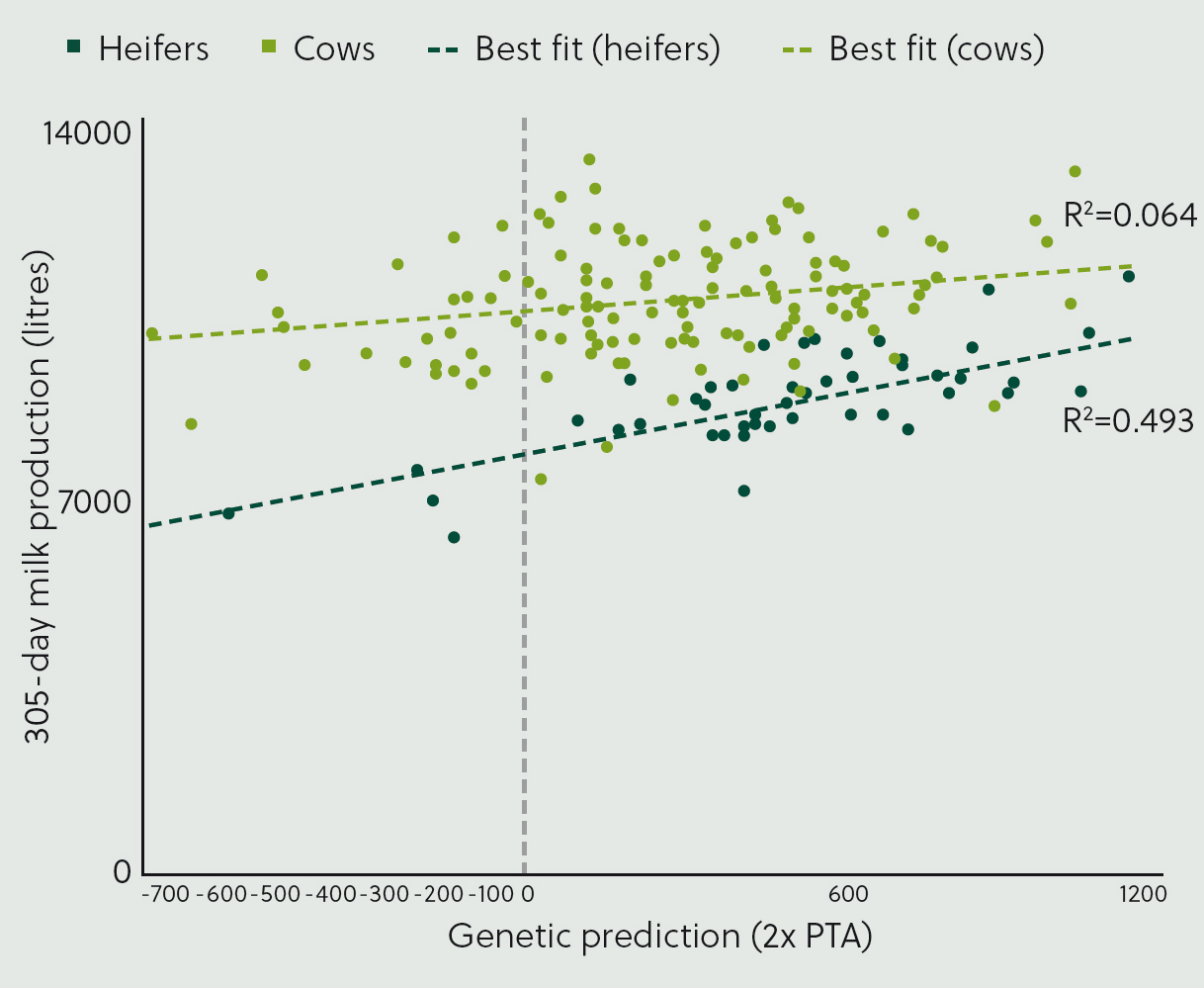
For cows, the data display a different trend. While the line of best fit is still positive, the gradient is less steep. This suggests that management factors are limiting the ability of older cows to express their genetic potential for milk production. Proactive herd health advice could help identify and address these management deficiencies. For instance, issues may stem from suboptimal nutrition, such as poor ration formulation, inadequate forage quality, insufficient concentrate availability or limited feedspace. Health problems in older cows, such as lameness, may also contribute to this limitation.
Fertility
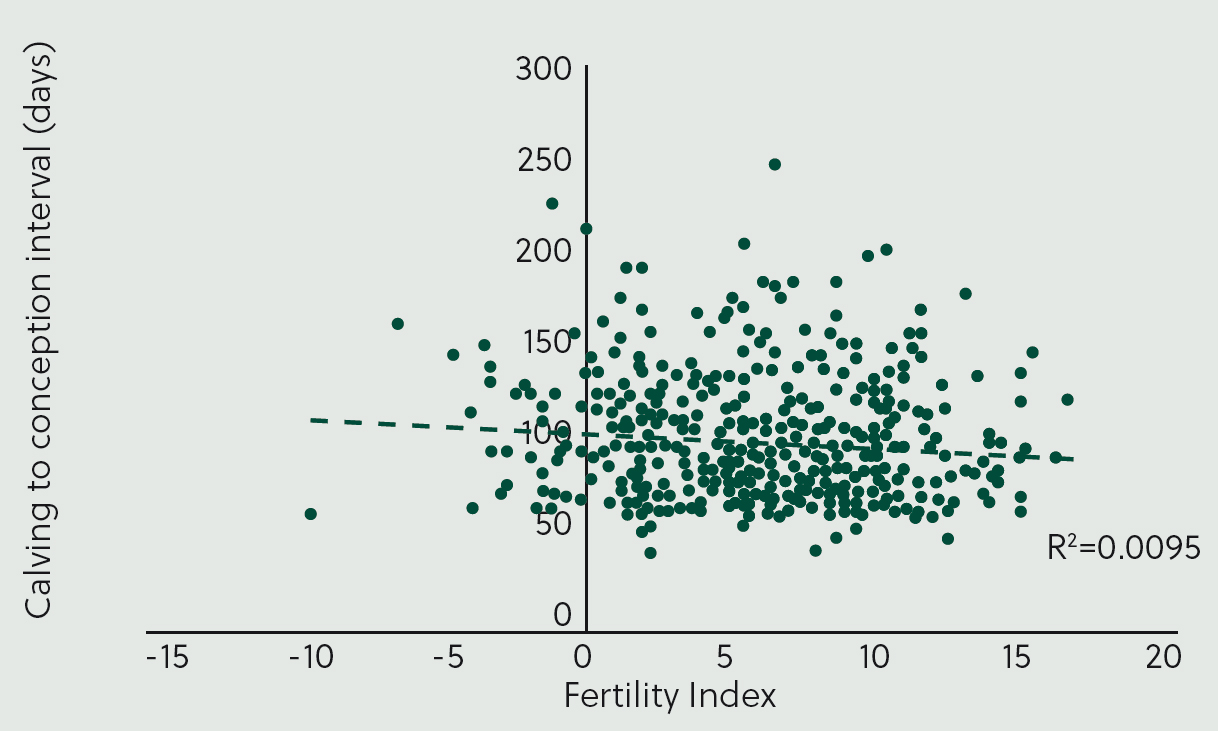
Figure 5 illustrates the association between Fertility Index and the calving-to-conception period for a dairy herd. The R2 value is 0.0095, indicating that variation in Fertility Index accounts for less than 1% of the variation in the calving-to-conception interval. This suggests that genetics has a minimal impact on fertility in this herd. To improve fertility performance, management changes are likely to be more effective than breeding for enhanced fertility.
Health
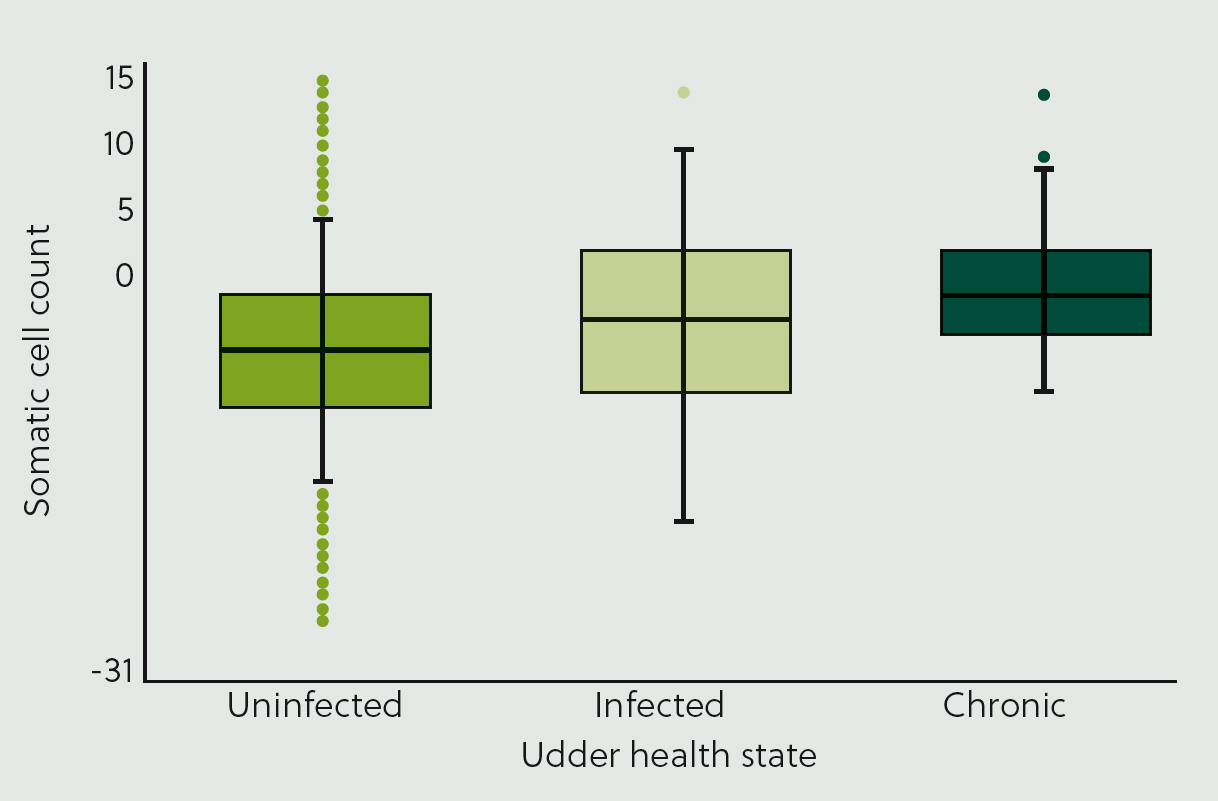
Somatic cell count (SCC) is a low heritability trait (Carlén et al, 2004), but breeding for improved SCC can still provide benefits. Figure 6 shows the distribution of genetic predictions for SCC based on the current udder health state, where lower scores are desirable. Cows with chronically high cell counts have the poorest genetic predictions for SCC, while cows that are currently uninfected have the best genetic predictions. This graph indicates that genetics for SCC are being expressed, suggesting that selective breeding could play a modest role in reducing cell counts in this herd.
Conclusions
The quality of genetic and performance data is improving, making this an exciting time to work in this field. Genomic testing is most cost-effective in unproven heifers and cows without sire or dam records, typically providing a net benefit to farmers over the animal's lifetime. Evaluating how genetics are being expressed on a farm is essential. If genetics are not being expressed, management is likely to be the limiting factor.


